Abstract
Pyrrolizidine alkaloids (PAs) are natural phytotoxins found in thousands of plant species around the world. They are probably the most common poisonous plants affecting livestock, wildlife and humans. The disease occurs almost entirely as a consequence of chronic poisoning and in general ends fatally. In the present study, PAs poisoning was investigated in a gazelle with hepatic encephalopathy associated with severe neurologic signs. The main clinical signs included head pressing, progressive depression and weakness, ataxia and reluctance to move, turn the head to the left and to paddle, hyperesthesia and decreased food intake. Histopathological examination revealed major lesions in the liver consisting of severe hepatocyte megalocytosis and hypertrophy with nuclei enlargement, mild bile duct hyperplasia, centriacinar fatty change and hepatocellular necrosis. Moreover, pulmonary congestion and edema with endothelium necrosis and alveolar septa thickening, severe congestion in vessels of the brain and meninges, and myocardial necrosis were observed.
Keywords: pyrrolizidine alkaloids, encephalopathy, gazella, megalocytosis
Introduction
Pyrrolizidine alkaloids (PAs) are naturally occurring compounds which are contained in about 3% of all flowering plants (Bull et al., 1968). They are probably the most common poisonous plants affecting livestock, wildlife and humans (Creeper et al., 1999; Stegelmeier et al., 1999). More than 660 pyrrolizidine alkaloids and N-oxide derivatives have been identified in over 6000 plants of the three families: Boraginaceae, Compositae (Asteraceae), and Legumionsae (Fabaceae), and about half of them exhibited toxic activities (Roeder, 2000; Stegelmeier et al., 1999).
Intake of pyrrolizidine alkaloid containing plants has poisoned humans and livestock worldwide, including horses (Creeper et al., 1999; de Lanux-Van Gorder, 2000), cattle (Dickinson et al., 1976), sheep and hamster (Huan et al., 1998), goat (Dickinson & King, 1978) and rat (Allen et al., 1975). The toxic effects of pyrrolizidine alkaloids gained further attention when experimental animals, particularly rats, dosed with these compounds developed liver tumors and pulmonary lesions (Chan et al., 2003; Fu et al., 2002). There are numerous reports of human poisoning caused by pyrrolizidine alkaloids (Chauvin et al., 1994; Stegelmeier et al., 1999). Pyrrolizidine alkaloids were found to contaminate such human food sources as wheat, milk and honey, and exposure through these routes is suspected to have caused worldwide human health problems (Stegelmeier et al., 1999).
Because of their abundance and potent toxicities, including hepatotoxicity and carcinogenicity, pyrrolizidine alkaloids received greatest attention (Fu et al., 2004). PAs are metabolized to highly reactive pyrroles, which are antimitotic cytotoxins. Hepatocyte nuclei are most commonly affected, causing irreversible liver damage (Stegelmeier et al., 1999). This paper describes for the first time clinical signs and histopathological features of PAs toxicity in different tissues of a gazelle (Gazella Subgutturosa).
Case history
In September 2014, a 1.5 year-old gazelle (Gazella Subgutturosa), which belonged to the Department of Enviroment, East Azarbayjan, Tabriz, Iran, was presented to the University of Tabriz, Veterinary Medical Teaching Hospital, with severe neurological signs . The main signs, which started in the morning, included head pressure, progressive depression and weakness, ataxia, hyperesthesia, decreased food intake, reluctance to move, turn the head to the left, or to paddle. The gazelle died in the evening, although supportive treatment was provided. Necropsy was performed and the appropriate samples were collected from different tissues (heart, spleen, lung, liver and brain) for histopathological studies. The tissue samples were fixed in 10% neutral buffered formalin, processed routinely, and embedded in paraffin wax. Sections (5 μm) were stained by hematoxylin and eosin and studied by light microscope.
Clinical findings
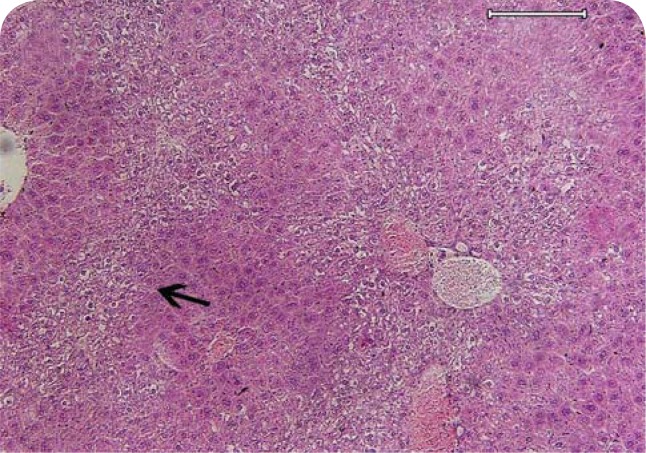
At necropsy, there was ascites, mild icterus and visceral organ congestion. The shape of the liver was normal but its size was somewhat enlarged. Histopathological examination revealed major lesions in the liver consisting of moderate congestion, hepatocyte megalocytosis with nucleus enlargement (Figure 1), mild bile duct hyperplasia, moderate to severe centriacinar zonal fatty change and mild focal hepatocellular necrosis (Figure 2). Moreover, Kupffer cell hyperplasia, mild focal hepatitis with neutrophil margination and sequestration was presented in the liver. Microscopically, there was a reactive liver. In the lung, severe pulmonary congestion and edema with endothelial necrosis and alveolar septa thickening was observed (Figure 3). There was also severe congestion and hyperemia in the brain and meninges. The heart showed mild focal myocardial necrosis. Lymphatic depletion was seen in the white pulp of the spleen (Figure 4).
Figure 1.
Pyrrolizidine alkaloid poisoning, liver, gazelle. Moderate congestion (arrow head), hepatocyte megalocytosis with nuclei enlargement (semi thin arrow), mild focal hepatitis (thick arrow) with neutrophil margination and sequestration (thin arrow) presented in the liver. (H&E; scale bar=60μm).
Figure 2.
Pyrrolizidine alkaloid poisoning, liver, gazelle. Moderate to severe centriacinar zonal fatty change, some of them showing mild focal hepatocellular necrosis (arrow) with moderate congestion of central veins. (H&E; scale bar=200μm).
Figure 3.
Pyrrolizidine alkaloid poisoning, lung, gazelle. Severe pulmonary congestion (thin arrow) and edema with alveolar septa thickening (thick arrow) was observed. (H&E; scale bar=200μm).
Figure 4.
Pyrrolizidine alkaloid poisoning, spleen, gazelle. Lymphatic depletion (arrow) was seen in white pulp. (H&E; scale bar=200μm).
Discussion
Pyrrolizidine alkaloids are highly toxic to many animal species, including most domestic livestock, and they have caused tremendous livestock loss (Creeper et al., 1999). Pyrrolizidine alkaloids, particularly those from plant species of the genera Senecio, Crotalaria, Heliotropium, and Amsinckia, were found to exhibit acute toxicity, chronic toxicity and genotoxicity (Fu et al., 2004).
The toxicity of a pyrrolizidine alkaloid containing plant depends on many factors, such as the amount of alkaloid that can be converted to reactive metabolites, the species exposed, age and sex of the animal intoxicated, the metabolic activity and the mitotic stage of its target cells (Stalker & Hayes, 2007).
Pigs and chickens are considered to be the most sensitive livestock species; cattle and horses are less so, yet they are still considered to be sensitive species. Sheep and goats are the least sensitive livestock species due to detoxification of PAs by rumen microbes and the liver (Stegelmeier et al., 1999). Sheep can graze out stands of Senecio that would be lethal to cattle (Stalker & Hayes, 2007). According to the history of the affected gazelle, which developed severe neurological signs in the morning and died in the evening, it appears to be a most sensitive livestock species, comparable to pigs. Young, still developing animals are most susceptible to PAs poisoning.
Most PAs are hepatotoxic because they are metabolically activated to dehydropyrrolizidine (DHP) in the liver. Monocrotaline is bioactivated in the lung and is therefore pneumotoxic. There are three common expressions of PAs poisoning. First, acute poisoning causes massive hepatotoxicity with hemorrhagic necrosis. The potency of hepatotoxicity and acute toxicity varies markedly among pyrrolizidine alkaloids (Mattocks & Cabral, 1984). Acute periacinar zonal necrosis occurs in animals ingesting large amount of PAs. Since these responsible plants are unpalatable, naturally occurring outbreaks of acute poisoning are generally restricted to circumstances when animals face starvation, such as grazing pastures affected by prolonged drought. After experimental exposure to high doses of PAs, acute hemorrhagic periacinar necrosis has been described. Second, phasic (usually seasonal) repetitive exposure to these PAs leads to hepatic atrophy with formation of regenerative nodules. Chronic poisoning takes place mainly in the liver, lungs, and blood vessels, and in some instances in kidneys, pancreas, gastrointestinal tract, bone marrow and brain (Mattocks & Cabral, 1984). These are the most common expressions of field exposure to Pas, with affected livers presenting a characteristic pattern of hepatocellular polyploidy known as megalocytosis. If seasonal exposure to these toxic alkaloids declines, a remarkable degree of recovery is possible (Stalker & Hayes 2007). Third, exposure over a long period of time causes cell enlargement (megalocytosis), veno-occlusion in liver and lungs, nucleus enlargement with increasing nuclear chromatin, loss of metabolic function, inhibition of mitosis, fatty degeneration, proliferation of biliary tract epithelium, liver cirrhosis, nodular hyperplasia and adenomas or carcinomas (Bull et al., 1968; Mattocks & Cabral, 1984; Roeder, 1995). Prolonged exposure exclusively to Heliotropium induces firm, fibrotic atrophic livers without nodular regeneration (Stalker & Hayes 2007). The histopathologic results of the present study corresponded more to the second form of expression of PAs poisoning, which is the most common expression of field exposure to PAs. At necropsy, the shape of the liver was normal but it showed a characteristic pattern of megalocytosis consisting of polyploidy hepatocytes with an extremely enlarged cytoplasm and nucleus. Hepatic megalocytosis (HM)-which is also known as megalocytic hepatosis – is often used as bioindication of exposure to such agents (Hinton et al., 1992). In one suggested interpretation, the failure of cell division in mitosis, is resulting in polyploidy hepatocytes with extremely enlarged cytoplasm and nucleus (Hinton et al., 1992).
In chronic PAs poisoning, there is usually proliferation of bile duct epithelial cells in the portal triads (Stalker & Hayes 2007), which in the presented pathological findings was only mild. There may be some periportal fibroplasia that varies with species and exposure; typically, it is minimal in sheep, moderate in horses and may be marked in cattle (Stalker & Hayes 2007). According to the pathological findings of the present study, it was minimal also in the gazzella. In fatal cases in cattle, the fibrotic livers can result in portal hypertension with ascites, severe mesenteric edema and diarrhea. In sheep, clinical signs may not be seen until after a second season of exposure. The shape of these affected livers is normal, but they are small, greyish yellow, fairly smooth and toughened by condensation of normal stroma rather than by fibroplasia (Stalker & Hayes 2007). Horses are susceptible to both acute and chronic toxicosis. Liver failure produced in horses by these alkaloids is similar to that seen in cattle. Horses are more likely to manifest signs of hepatic encephalopathy with head-pressing and compulsive walking; in some places these nervous signs give rise to colloquial names such as “walkabout” and “walking disease” (Stalker & Hayes 2007). Clinical signs of PAs poisoning in the gazella appear to be similar to those seen in horses.
Administration of a high dose of Cynoglossum officinale resulted in severe liver disease within 7 days after dosing with elevated serum enzymes, altered bile acid metabolism and extensive hepatocellular necrosis with minimal periportal fibrosis and bilary hyperplasia and little or no megalocytosis. Administration of a low dose to horses for 14 days resulted in transient clinical depression and weight loss, transient elevations of serum enzymes and bile acids and minimal periportal hepatocellular necrosis with fibrosis, developing extensive megalocytosis by week 14. Megalocytosis became the most prominent change 6 months after exposure (Stegelmeier et al., 1996). Liver failure, hepatoencephalopathy and histologic features produced in the gazelle by PAs were similar to those seen in the horse with administration of a low dose for 14 days. In this study, minimal periportal fibrosis and bile duct hyperplasia, extensive megalocytosis and periacinar zonal hepatocellular fatty change and necrosis was observed, which are more similar to changes seen in sheep and horses.
Moreover, in the present study, samples of the lung showed severe pulmonary congestion with pulmonary endothelium necrosis and thickening of alveolar septa. These lesions are in agreement with results obtained later. Mattocks and Cabral (1984) reported that monocrotaline (one of PAs metabolites) is bioactivated in the lung and is therefore pneumotoxic. The heart showed myocardial necrosis. There was also severe congestion in vessels of the brain and meninges. Lymphatic depletion was seen in the spleen. In previous studies, heart and spleen lesions have not been reported.The metabolites of PAs affect tissues other than the liver. Death in some instances may be due to renal damage and in others to pulmonary vascular and interstitial lesions. Variation in the source of the toxin and in the species of the target animal accounts for the differences in susceptibility of the different tissues. Alkaloids from Crotalaria affect the widest range of tissues in most animals (Stalker & Hayes 2007). Pulmonary changes seen in horses exposed to some Crotaria spp may include hyperplasia of bronchioalveolar epithelium, congestion, septal fibrosis and emphysema. Renal tubular lining cells and glomerular epihtelial cells also may be individually enlarged in pigs (Bildfell, 2013). Unfortunately, in this study, the kidney was not studied.
Generally in the gazelle, according to the results of the present paper, susceptibility to PAs poisoning, clinical signs and post mortem findings are similar to those seen in pigs, horses and sheep, respectively. PAs presented in this region like alkaloids from Crotalaria affect the widest range of tissues in the gazelle. As regards this region (Kaghaz-kanan in East Azabayjan, Iran), it is the last habitat of the Gazella Subgutturosa and detection of PAs seems to be mandatory for the survival of these animals.
Acknowledgements
The authors are grateful to the School of Veterinary Medicine, Tabriz University, Tabriz, Iran for the financial support.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
REFERENCES
- Allen JR, Hsu IC, Carstens LA. Dehydroretronecine-induced rhabdomyosarcomas in rats. Cancer Res. 1975;35:997–1002. [PubMed] [Google Scholar]
- Bildfell R. Overview of Pyrrolizidine Alkaloidosis, Merck Veterinary Mannual. 2013. http://www.merckmanuals.com.
- Bull LB, Culvenor CC, Dick AJ. Their Chemistry, Pathogenicity and Other Biological Properties. Amsterdam: North-Holland; 1968. The Pyrrolizidine Alkaloids. [Google Scholar]
- Chan PC, Haseman JK, Prejean J D, Nyska A. Toxicity and carcinogenicity of riddelliine in rats and mice. Toxicol Lett. 2003;144:295–311. doi: 10.1016/s0378-4274(03)00240-6. [DOI] [PubMed] [Google Scholar]
- Chauvin P, Dillon JC, Moren A. An outbreak of Heliotrope food poisoning, Tadjikistan, November 1992–March 1993. Sante. 1994;4:263–268. [PubMed] [Google Scholar]
- Creeper JH, Mitchell AA, Jubb TF, Colegate SM. Pyrrolizidine alkaloid poisoning of horses grazing a native heliotrope (Heliotropium ovalifolium) Aust Vet J. 1999;77:401–402. doi: 10.1111/j.1751-0813.1999.tb10318.x. [DOI] [PubMed] [Google Scholar]
- de Lanux-Van Gorder V. Tansy ragwort poisoning in a horse in southern Ontario. Can Vet J. 2000;41:409–410. [PMC free article] [PubMed] [Google Scholar]
- Dickinson JO, Cooke MP, King RR, Mohamed PA. Milk transfer of pyrrolizidine alkoloids in cattle. J Am Vet Med Assoc. 1976;169:1192–1196. [PubMed] [Google Scholar]
- Dickinson JO, King RR. The transfer of pyrrolizidine alkaloids from Senecio jacobaea into the milk of lactating cows and goats. In: Keeler R. F, VanKampen K. R, James L. F, editors. Effects of Poisonous Plants on Livestock. New York: Academic Press; 1978. [Google Scholar]
- Fu PP, Yang YC, Xia Q, Chou MW, Cui YY, Lin G. Pyrrolizidine alkaloids—tumorigenic components in Chinese herbal medicines and dietary supplements. J Food Drug Anal. 2002;10:198–210. [Google Scholar]
- Fu PP, Xia Q, Lin G, Chou MW. Pyrrolizidine Alkaloids—Genotoxicity, Metabolism,Enzymes, Metabolic Activation and Mechanisms. Drug Metab Rev. 2004;36(1):1–55. doi: 10.1081/dmr-120028426. [DOI] [PubMed] [Google Scholar]
- Hinton DE, Bauman PC, Gardner GR, Hawkins WE, Hendricks JD, Murchelano RA, Okihiro MS. Histologic biomarkers. In: Huggett R.J, Kimerle R.A, Mehrle P.M Jr, Bergman H.L, editors. Biomarkers of anthropogenic stress. Boca Raton: Lewis Publ.; 1992. pp. 155–209. [Google Scholar]
- Huan JY, Miranda CL, Buhler DR, Cheeke PR. The roles of CYP3A and CYP2B isoforms in hepatic bioactivation and detoxification of the pyrrolizidine alkaloid senecionine in sheep and hamsters. Toxicol Appl Pharm. 1998;151:229–235. doi: 10.1006/taap.1998.8482. [DOI] [PubMed] [Google Scholar]
- Mattocks AR, Carbal JRO. Carcinogenicity of some pyrrolic pyrrolizidine alkaloid metabolites and analogues. Cancer Letter. 1984;17:61–66. doi: 10.1016/0304-3835(82)90109-4. [DOI] [PubMed] [Google Scholar]
- Roeder E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Arch Pharm. 1995;50:83–98. [PubMed] [Google Scholar]
- Roeder E. Medicinal plants in China containing pyrrolizidine alkaloids. Arch Pharm. 2000;55:711–726. [PubMed] [Google Scholar]
- Stalker MG, Hayes MA. Pyrrolizidine alkaloids; Chronic hepatotoxicity: plant- derived and environmental toxins. In: Maxie M. G, editor. Pathology of Domestic Animals. 5th ed. (Courtesy of University of Melbourne); 2007. pp. 373–376. [Google Scholar]
- Stegelmeier BL, Gardner DR, James LF, Molyneux RJ. Pyrrole detection and the pathologic progression of Cynoglossum officinale poisoning in horses. J Vet Diagn Invest. 1996;8:81–90. doi: 10.1177/104063879600800113. [DOI] [PubMed] [Google Scholar]
- Stegelmeier BL, Edgar JA, Colegate SM, Gardner DR, Schoch TK, Coulombe RA, Molyneux RJ. Pyrrolizidine alkaloid plants, metabolism and toxicity. J Nat Toxins. 1999;8:95–116. [PubMed] [Google Scholar]