Figure 2. Peli1 co-localizes and interacts with BubR1 at mitotic phase of cell cycle.
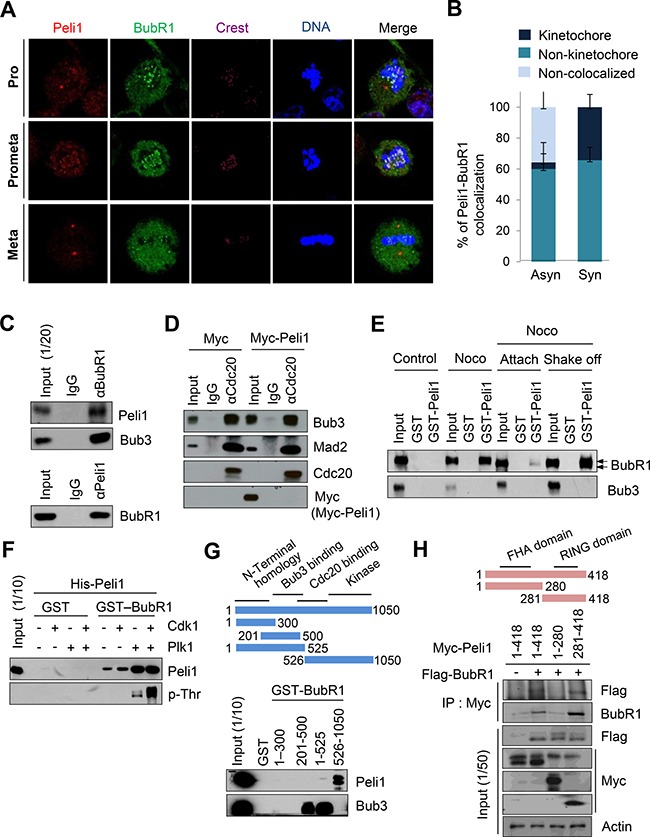
(A) Immunofluorescence images of HeLa cells at the mitotic phase are shown. Cells were stained with anti-BubR1 antibody (green), anti-Peli1 antibody (red) or Hoechst (blue), and examined by confocal microscopy. (B) The graph represents the percentages of Peli1 co-localized with BubR1 at kinetochores in asynchronized or synchronized cells. At least 30 of each cell type were analysed in three independent experiments. Data are presented as mean values (n = 3). (C) Ramos cells were synchronized by treating nocodazole for 18 hr. Cell lysates were then immunoprecipitated with anti-BubR1 (upper) or anti-Peli1 antibody (lower) and then immunoblotted with anti-Peli1 and anti-Bub3 (positive control) or anti-BubR1 antibody. (D) HeLa cells were transfected with Myc (control) or Myc-Peli1 expression plasmids. At 24 hr post-transfection, cells were treated with nocodazole (200 ng/ml) for 24 hr and harvested for immunoprecipitation with an anti-Cdc20 antibody. (E) HeLa cells were synchronised using nocodazole and then separated into attached (Attach) and floating populations by mitotic shake-off (Shake-off). Cell lysates from asynchronous (control) and synchronous HeLa cells were incubated with beads bound to GST or GST-Peli1. Upper arrowheads indicate the hyperphosphorylated form of BubR1. (F) Bead-bound GST and GST-BubR1 were reacted with the Plk1 or Cdk1/Cyclin B kinase in the presence of unlabelled ATP. GST-BubR1 proteins phosphorylated or left unphosphorylated in vitro were incubated with purified His-Peli1. In vitro phosphorylated GST-BubR1 was immunoblotted with anti-Peli1 and anti-phosphothreonine (p-Thr) antibodies. (G) Structural schematic of BubR1 showing its NH2-terminal homology and Bub3-binding, Cdc20-binding, and kinase domains. Ramos cells were synchronised using nocodazole, and lysates were incubated with GST alone or with a series of BubR1 deletion mutants fused to GST. Bound proteins were resolved by SDS-PAGE and immunoblotted with an anti-Peli1 or anti-Bub3 (positive control) antibody. (H) Structural schematic diagram of Peli1 showing N-terminal forkhead-associated (FHA) domain and C-terminal RING domain with the characteristic feature of the RING class of E3 ubiquitin ligases. HeLa cells were transfected with Myc-Peli1 (full-length) (amino acids 1-418) or deletion mutants (amino acids 1-280 or 281-418) in combination with Flag-BubR1 expression plasmids. At 24 hr post-transfection, cells were treated with nocodazole (200 ng/ml) for an additional 24 hr and harvested for immunoprecipitation with an anti-Myc antibody and immunoblotting with anti-Flag antibody.
