Figure 3. Peli1 directly down-regulates the stability of BubR1 by K48-mediated polyubiquitination.
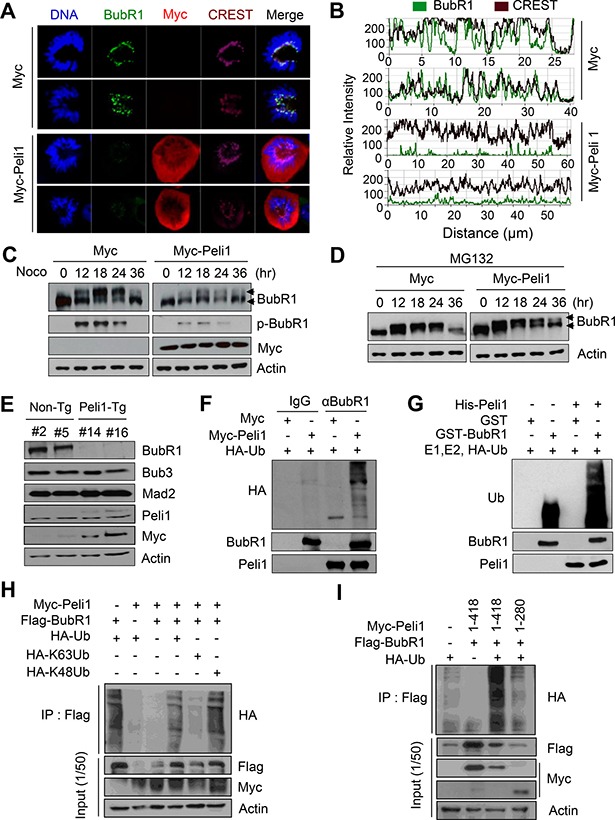
(A) HeLa cells were transiently transfected with a Myc or Myc-Peli1 expression plasmid, stained with an anti-BubR1 (green), an anti-Myc (red) antibody, the CREST serum (purple), or Hoechst (blue), and examined by confocal microscopy. Results are representative of three independent experiments. (B) The relative intensity of BubR1 and proteins targeted by CREST serum was compared in control and Peli1-overexpressing cells. (C, D) HeLa cells were transfected with a Myc or Myc-Peli1 expression plasmid, at 24 hr post-transfection, treated with nocodazole for different time-points, and further cultured in absence or presence of 25 μM MG132 for 5 hr. Harvested cellular lysates were immunoblotted with an anti-BubR1 antibody. Upper arrowheads indicate the hyperphosphorylated form of BubR1. (E) Splenocytes were isolated from non-Tg and Peli1-Tg mice and stimulated by lipopolysaccharides. At 24 hr post-treatment, splenocytes were harvested, lysed, and subjected to immunoblotting with the indicated antibodies. (F) HeLa cells were cotransfected with Myc or Myc-Peli1 and an HA-ubiquitin expression plasmid and then cultured in presence of nocodazole and MG132 as described above. The BubR1 immunocomplex was immunoprecipitated from transfected cell lysates with an anti-BubR1 antibody and subjected to immunoblotting with anti-HA, anti-BubR1 and anti-Peli1 antibodies. (G) Purified GST or GST-BubR1 was incubated with purified His-Peli1 in conjunction with E1, E2, and hemagglutinin-tagged ubiquitin (HA-Ub) enzymes. Reaction mixtures were immunoblotted with anti-ubiquitin and anti-Peli1 antibodies. (H) HeLa cells were transfected with Myc-Peli1, Flag-BubR1 and HA-Ub, HA-K63Ub or HA-K48Ub expression plasmids and then cultured in presence of nocodazole and MG132. Cells were harvested for immunoprecipitation with anti-flag antibody and immunoblotting with anti-HA antibody. (I) HeLa cells were transfected with Myc-Peli1 (full-length) (amino acids 1-418) or C-terminal RING domain deletion mutant (amino acids 1-280) in combination with Flag-BubR1 and HA-Ub expression plasmids. Cells were then cultured in presence of nocodazole and MG132 and harvested for immunoprecipitation with anti-Flag antibody and immunoblotting with anti-HA antibody.
