Fig. 5.
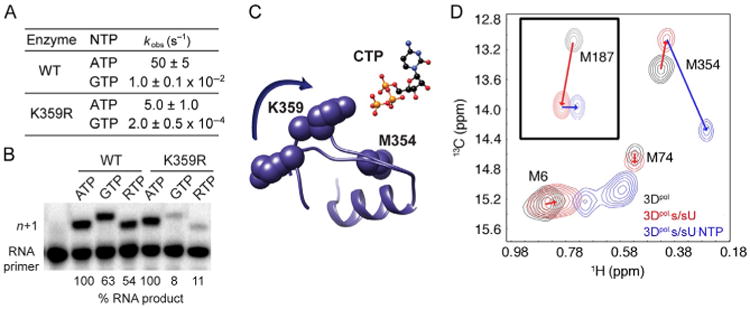
PV RdRp Lys-359 in structural motif D is a determinant of RdRp catalytic efficiency and fidelity. (A and B) K359R PV RdRp is slower and more faithful than the WT lysine-containing enzyme. RTP stands for ribavirin triphosphate. (C) Motif D changes conformation upon nucleotide binding. (D) The Met-354 resonance serves as a reporter of the catalytically competent complex. A substantial change in this resonance is only observed when the correct NTP is bound. Panels (A and B): Adapted from S.A. Weeks, C.A. Lee, Y. Zhao, E.D. Smidansky, A. August, J.J. Arnold, C.E. Cameron, A polymerase mechanism-based strategy for viral attenuation and vaccine development, J. Biol. Chem. 287 (38) (2012) 31618–31622. Panel (D): Adapted with permission from X. Yang, J.L. Welch, J.J. Arnold, D.D. Boehr, Long-range interaction networks in the function and fidelity of poliovirus RNA-dependent RNA polymerase studied by nuclear magnetic resonance, Biochemistry 49 (43) (2010) 9361–9371. Copyright (2010) American Chemical Society.
