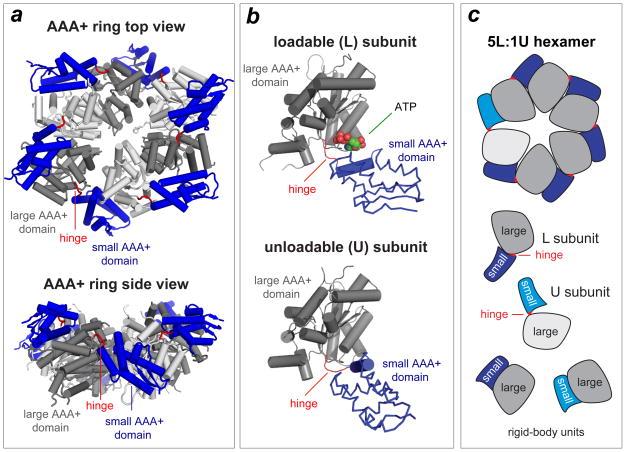
Figure 3. ClpX ring structure.
(a) Views of the AAA+ ring of E. coli ClpX (pdb 3HWS). In each subunit, the large AAA+ domain is coloured dark or light grey and the small AAA+ domain is coloured purple. Hinges between the large and small domains of each subunit are coloured red. (b) Subunits can adopt either a loadable (L) or an unloadable (U) conformation. In L subunits, ATP binds in a cleft between the large and small AAA+ domains. In U subunits, rotation of the small domain destroys the binding pocket. (c) Cartoon of a 5L:1U ClpX ring showing how six rigid-body units connected by six hinges are created by packing between the small AAA+ domain of a subunit (coloured blue) and the large AAA+ domain of a neighbouring subunit (dark grey for L subunits; light grey for U subunits). Because the ring is topologically closed, changes in the conformation of any single hinge — caused by ATP binding, ATP hydrolysis or product release — propagates around the ring.