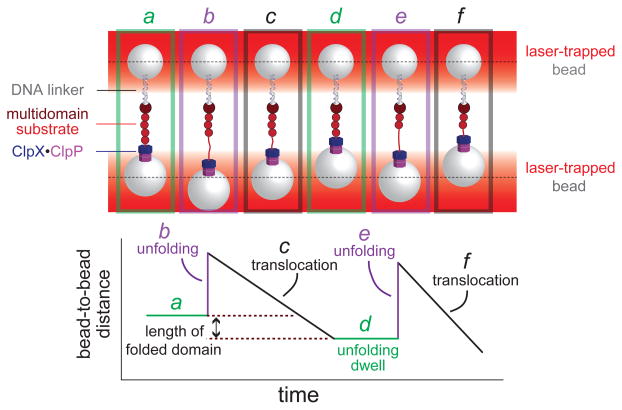
Figure 4. Single-molecule force spectroscopy of ClpXP.
(a) Cartoon of an optical-trapping experiment. Micron-sized beads, trapped by infrared lasers, are tethered to either ClpXP or a multi-domain substrate via a DNA linker. When ClpXP engages the substrate, ATP-fuelled mechanical activity can be monitored by measuring bead movements relative to the centre of laser focus (dotted lines). (b) ClpXP unfolding of an individual substrate domain (panels a2 and a5) results in an increase in bead-to-bead distance. Translocation of the substrate (panels a3 and a6) results in a decrease in bead-to-bead distance that corresponds to the length of the translocated domain. Periods of no movement are dwells (panels a1 and a4) in which ClpXP tries to unfold the next native domain in the substrate.