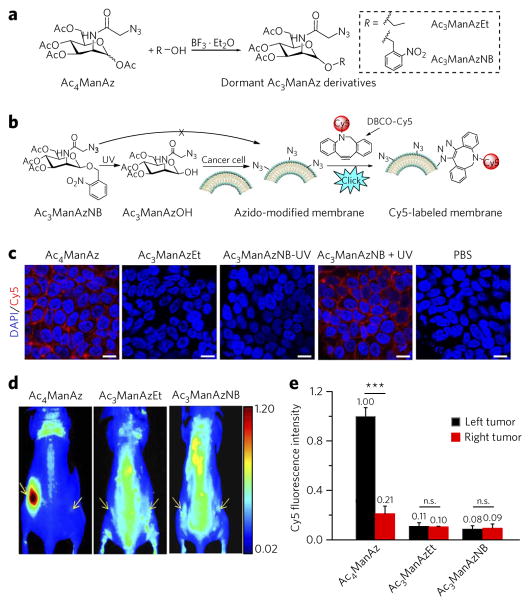
Figure 2. Replacing the C1–OAc of Ac4ManAz with a cleavable ether bond–enabled controlled metabolic cell labeling in vitro and in vivo.
(a) Synthetic route of dormant Ac3ManAz derivatives including Ac3ManAzEt and UV-responsive Ac3ManAzNB. (b) Schematic illustration of UV-irradiation-activated metabolic labeling of Ac3ManAzNB and subsequent detection of the expressed azido groups by DBCO–Cy5 via click chemistry. (c) Confocal laser scanning microscopy (CLSM) images of LS174T colon cancer cells after incubation with Ac4ManAz (50 μM), Ac3ManAzEt (50 μM), Ac3ManAzNB (50 μM) − UV, Ac3ManAzNB (50 μM) + UV or PBS for 72 h and labeling with DBCO–Cy5 (50 μM, red) for 1 h. The cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). Scale bars, 10 μm. (d, e) Ac4ManAz, Ac3ManAzEt or Ac3ManAzNB (25 mM, 20 μl) was injected intratumorally (i.t.) to the left LS174T tumors of athymic nude mice once daily for three consecutive days (days 1–3), and the right LS174T tumors were injected i.t. with PBS as controls. DBCO–Cy5 (5 mg/kg) was i.v. injected on day 4. (d) In vivo fluorescence imaging of mice from different groups at 48 h p.i. of DBCO–Cy5. Tumors are shown by yellow arrows. (e) Ex vivo Cy5 fluorescence intensity of the tumor tissues harvested at 48 h p.i. of DBCO–Cy5. Data are presented as mean ± s.e.m. (n = 3) and analyzed by one-way ANOV A (Fisher; 0.01 < *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). n.s., not significant. Data represent results from at least three experiments.