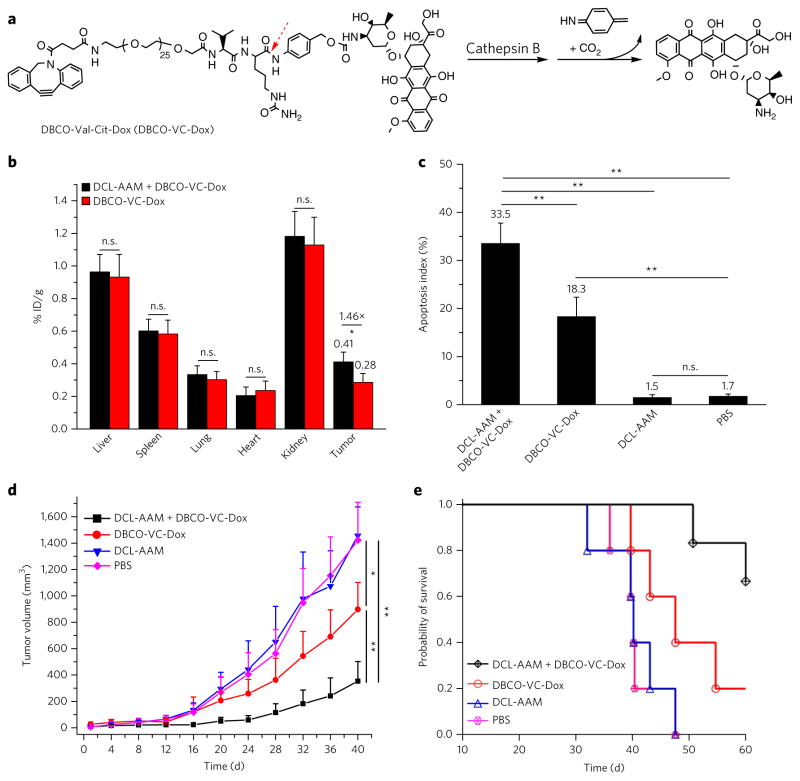
Figure 5. DCL-AAM-mediated tumor labeling improved antitumor efficacy of DBCO–drug conjugate against primary LS174T colon tumor and MDA-MB-231 triple-negative breast tumor models.
(a) Structure of DBCO-VC-Dox with a cathepsin-B-cleavable linker that consists of a dipeptide (Val–Cit), a self-immolative p-aminobenzylcarbamate and a short polyethylene glycol segment (1 kDa). (b–d) Short-term efficacy study in athymic nude mice bearing subcutaneous LS174T tumors. DCL-AAM (60 mg/kg) was injected i.v. once daily for 3 d (days 0, 1 and 2). Subsequently, DBCO-VC-Dox (10 mg/kg in Dox equivalent) was injected i.v. on day 3. Tumors were harvested for analysis at 48 h p.i. of DBCO-VC-Dox (on day 5). (b) Retained drugs in tissues of drug-treated mice (n = 3). % ID/g, percent of injected dose normalized by gram of tissue. (c) Quantification of TUNEL stains of LS174T tumors from mice treated with DCL-AAM + DBCO-VC-Dox, DBCO-VC-Dox, DCL-AAM and PBS, respectively (20 sections per tumor, n = 6). (d, e) Long-term antitumor efficacy study in nude mice bearing subcutaneous MDA-MB-231 tumors. DCL-AAM (60 mg/kg) was injected i.v. on days 0, 1 and 2. Subsequently DBCO-VC-Dox (12 mg/kg in Dox equivalent) was injected i.v. on days 3, 7 and 11. (d) Average MDA-MB-231 tumor volume of each group of mice (DCL-AAM + DBCO-VC-Dox and DBCO-VC-Dox group, n = 6; DCL-AAM and PBS group, n = 5) over the course of the efficacy study. (e) Kaplan–Meier plots for all groups. Loss of mice was a result of treatment-related death or euthanasia after the predetermined end point was reached. All the numerical data are presented as mean ± s.e.m. and analyzed by one-way ANOV A (Fisher; 0.01 < *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).