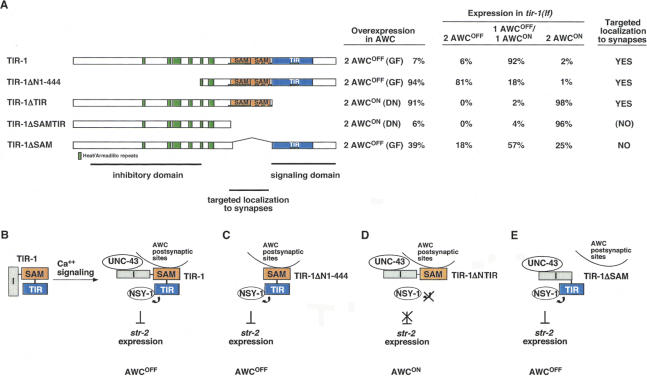
Figure 5.
Identification of TIR-1 functional domains. There are three inferred functional domains in the TIR-1 protein: a regulatory region at the N terminus, SAM domains for targeted localization to synapses, and a TIR domain for activation of signaling. (A) Structure-function analysis of TIR-1. (Far left) Diagram of mutated TIR-1 proteins tested for biological activity. (Left) Overexpression of transgenes in wild-type animals. “Gain-of-function” transgenes were injected into wild-type at 5–7 ng/μL. “Dominant-negative” transgenes were injected into wild-type at 35 ng/μL. Two lines were examined for each transgene; in all cases, both lines gave similar results. For overexpression experiments, n =63–141 animals. (Center) Rescue of tir-1(tm1111lf) animals. Two lines were examined for each transgene; in all cases, both lines gave similar results. For rescue experiments, n =73–107 animals. (Right) Subcellular localization of tagged protein. TIR-1ΔSAMTIR was expressed poorly, so it is possible that it is present at synapses at a level too low to be detected with the RFP marker. (B) Model for TIR-1 functions. The N-terminal inhibitory domain (I) could mask the TIR signaling domain. Ca++ signaling through UNC-43 stimulates TIR-1-dependent activation of NSY-1 at the synapse. (C) When the N-terminal inhibitory domain is deleted, TIR-1ΔN1-444 can activate NSY-1 independently of Ca++ signaling, leading to a strong gain-of-function 2 AWCOFF phenotype. (D) When the TIR signaling domain is deleted, TIR-1ΔTIR competes with wild-type TIR-1 for UNC-43 or other activators at the synapse, leading to a 2 AWCON dominant-negative effect. (E) TIR-1ΔSAM, deleting the synaptic localization domain, is not targeted to the synapse and has a mild gain of function phenotype when overexpressed.