Figure 10.
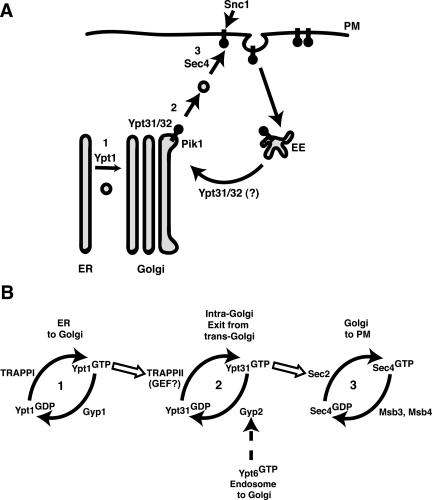
(A) Snc1p trafficking to the plasma membrane (PM) in yeast requires Golgi and early endosome (EE) function. Inactivation of Pik1p or Ypt31p causes Snc1p to accumulate intracellularly. We propose that the primary role for Pik1p/PtdIns(4)P is in maintaining the structural integrity and molecular identity of the trans-Golgi and that unidentified multivalent adaptor proteins may be recruited by Pik1p/PtdIns(4)P and/or activated Ypt31/32p for the coordination of cargo-loading, budding, and/or fission at the trans-Golgi. Alternatively, Ypt31/32p may also participate in retrograde traffic of recycled cargoes by regulating early endosome-to-Golgi transport. (B) The orderly cascade of yeast exocytic rab-GTPases. The cis-Golgi rab-GTPase, Ypt1p and the multicomponent complex, TRAPPII act upstream to the trans-Golgi rab-GTPase, Ypt31p, possibly leading to its activation. Activated, GTP-bound Ypt31p interacts with Sec2p, the GEF for Sec4p (Ortiz et al., 2002), whereas activated-Ypt6p may recruit Gyp2p (Siniossoglou and Pelham, 2001), which inactivates Ypt31p, allowing the rab-GTPase cycle of activation/inactivation at the Golgi to continue.
