Abstract
Antifungal prophylaxis is routinely given to patients with hematologic malignancies at high risk for invasive fungal infections (IFI), yet breakthrough IFI may still occur. Posaconazole emerged as an excellent alternative for fungal prophylaxis in high-risk patients. There is limited data about pharmacokinetics and plasma concentrations of posaconazole when given as prophylaxis in patients with hematologic malignancies. We recruited 20 adult patients for prospective, open label trial of posaconazole given as a prophylaxis in patients with newly diagnosed acute myeloid leukemia (AML) undergoing induction chemotherapy or first salvage therapy. The median age of all patients was 65 years and received prophylaxis for a median of 38 days (range: 5 to 42 days). Ten patients (50%) completed 42 days on posaconazole prophylaxis. Median plasma posaconazole levels showed no statistical difference across gender, body surface area, patients developing IFI, and patients acquiring grade 3 or 4 elevation of liver enzymes. However, there was an overall trend for higher trough concentrations among patients with no IFI than those with IFI. Pharmacokinetics of posaconazole varies from patient to patient, and AML patients receiving induction chemotherapy who never develop IFI tend to have higher plasma concentrations after oral administration of posaconazole.
Keywords: Posaconazole, Acute leukemia, Antifungal prophylaxis
1. Introduction
Antifungal prophylaxis is routinely given to patients with hematologic malignancies at high risk for invasive fungal infections (IFI). However, breakthrough IFI may occur still in some patients. The significance and causes of breakthrough IFI is not well known. Factors contributing to the occurrence of breakthrough IFI include the presence of severe immunosuppression, development of microbiological resistance, poor compliance of the patient to therapy, or inadequate antifungal blood levels.
Several regimens that include azoles, polyenes and echinocandins have been used for antifungal prophylaxis in high risk patients[1–4]. Because of its broad spectrum and oral availability, posaconazole emerged as an excellent alternative for fungal prophylaxis, and has been approved by the FDA for antifungal prophylaxis in high-risk patients [5–7]. The recommended dose of posaconazole for fungal prophylaxis is 200 mg oral suspension three times per day. However, posaconazole absorption is substantially affected by gastric pH, prandial state and timing of dose administration relative to meal[8]. The drug requires the dose to be given with or after high-fat meal or with nutritional supplements. If a patient cannot eat a full meal, posaconazole may be taken with an acidic carbonated beverage (e.g. ginger ale)[9]. Previous studies done in HSCT recipients and in patients with febrile neutropenia have shown that posaconazole achieves adequate plasma concentrations after oral administration[10]. Demographic variables such as race, sex, body weight and age do not have a clinically relevant effect on plasma concentrations. Additionally, a clear association between a divided dose regimen (four times daily; QID) or co-administration with a carbonated beverage with increased posaconazole exposure in healthy subjects has been demonstrated when compared with a twice daily (BID) regimen[11].
There is limited data available about pharmacokinetics and plasma concentrations of posaconazole when given as prophylaxis in patients with hematologic malignancies undergoing high-dose chemotherapy. The food requirement required for optimal absorption may not be possible in patients with hematologic malignancies due to the presence of severe/moderate mucositis, serious defects in absorption and altered taste which may lead to breakthrough fungal infections [12–14]. Therefore, several concerns have been raised about its absorption and pharmacokinetic profile in this high-risk group of patients.
2. Methods
We conducted a prospective, open label trial of posaconazole given as a prophylaxis in patients with newly diagnosed acute myeloid leukemia (AML) or high-risk myelodysplastic syndrome (HR-MDS) undergoing induction chemotherapy or first salvage therapy. The primary objective was to study the plasma pharmacokinetics of posaconazole in patients with newly diagnosed AML or HR-MDS undergoing induction chemotherapy or relapsed or refractory patients receiving salvage chemotherapy. The secondary objective was to evaluate the safety of posaconazole given as prophylaxis. Posaconazole was given as an oral suspension at 200 mg three times per day for a maximum of 42 days beginning within 24 hours after the last dose of chemotherapy if the chemotherapy included a CYP3A4 pathway. If the chemotherapy did not have a CYP3A4 pathway, treatment was given concomitant with chemotherapy and started as indicated by the treating physician. Each dose was to be administered with a full meal or immediately (+/− 20 minutes) following a full meal or liquid nutritional supplement. If a patient could not eat a full meal, an option to take posaconazole with an acidic carbonated beverage (e.g. ginger ale) was given to the patient.
Prophylaxis continued until at least one of the following occurred: a) 42 days elapsed from the start of therapy, or b) until hematologic response was assessed (Response, defined as achievement of complete remission, or resistance), or c) development of possible, probable or proven invasive fungal infections (according to EORTC/MSG criteria)[15], or d) development of unacceptable drug toxicity, or e) withdrawal from study participation whether by patient’s or investigator’s decision, or f) death. Posaconazole was provided at no cost to the patients participating in the study.
2.1 Patients
From January 2010 through August 2010, we recruited 20 adult patients with AML or HR-MDS undergoing induction or first salvage chemotherapy. Salvage chemotherapy is defined as the therapy given to patients that achieved remission and relapsed or patients who were refractory to initial frontline chemotherapy. All patients were required to be able to take oral medications and agreed to medically approved forms of contraception. Patients on prior antifungal prophylaxis with voriconazole, itraconazole or fluconazole were eligible if they completed a 3-day wash-out period if on voriconazole, 14-day wash-out if on itraconazole, or 7-day wash-out period if on fluconazole. The study was approved by the institutional review boards (IRB) at MD Anderson Cancer Center. Written informed consent was obtained from all participants and patients were registered with the Data Management Office CORE system.
Exclusion criteria included patients with history of anaphylaxis attributed to azole compounds, patients with clinical or other evidence that indicated that they have proven or probable invasive fungal infection prior to enrollment (EORTC criteria)[15], a baseline total bilirubin level >3 times the upper normal limit of normal (ULN) (i.e. > 3.0 mg/dl), baseline SGPT >5 times ULN, baseline creatinine levels >3.0 times ULN, baseline QTc >450 msec, and patients receiving any medication that is contraindicated with the use of posaconazole.
2.2 Pharmacokinetic samples
Pharmacokinetic samples (1.0 ml of plasma using EDTA) were obtained from every patient on day 1, day 3 and day 10 of prophylaxis. A total of 15 samples (3 ml each sample) per patient were obtained, with TID dosing planned around standard meal times.
On day 1 and day 3 of prophylaxis sampling was done pre dose (0), and at 3, 5, 10 and 24 hours (±10 minutes) post dose from the time of the first dose of the day. On day 10 of prophylaxis sampling was done pre dose (0), and at 3, 5, and 10 hours (±10 minutes) post dose from the time of the first dose of the day. Blood samples were sent for measurement of posaconazole levels to Fungus Testing Laboratory, Department of Pathology The University of Texas Health Science Center (7703 Floyd Curl Dr. San Antonio, Texas 78229-7750).
Posaconazole levels were measured by a validated high performance liquid chromatography (HPLC) assay[16–17]. The linear range for this assay is from 125 – 5000 ng/ml, with the inter-day coefficient of variation ranging between 13.8% to 14.5% for the runs conducted in this study.
2.3 Evaluation and Follow up
A complete blood count (CBC) and differential, total bilirubin, SGPT (or SGOT), alkaline phosphatase, and creatinine were performed at baseline in all patients. Chest X-ray was performed within 1 week of starting therapy. If patient was febrile and/or presented with signs and symptoms of respiratory infections (lungs or sinus), computed tomography was performed within 72 hours of the start of study drug. Serum galactamonnan (GM) index test was assessed within 2 days of initiation of antifungal therapy. Diagnosis of proven, probable or possible IFI were done following the standard EORTC criteria for IFI. One electrocardiogram was performed within 7 days from the start of study drug.
During study, patients were provided with a food diary to report the time the dose was taken in relationship to the meal and to describe what they ate or drank at the meal. Total bilirubin, SGPT or SGOT, and creatinine were monitored weekly. All patients received standard antibacterial and antiviral prophylaxis but no concomitant antifungal therapy was allowed while the patients was receiving study drug.
2.4 Statistics
We used the analysis of variance to evaluate the effect of various covariates on posaconazole concentrations such as gender, median body surface area (BSA) (<1.9 m2 vs > 1.9 m2), IFI (yes vs no) and grade 3 or 4 liver dysfunction (yes vs no). All data are reported as mean ± SD or median (range) unless otherwise indicated. A p value < 0.05 was considered as statistically significant.
3. Results
Twenty patients were enrolled in the study. The baseline patient characteristics are shown in table 1. The median age of all patients was 65 years and all but one patient were receiving their first induction chemotherapy, either with a clofarabine-based regimen or a cytarabine-based regimen.
Table 1.
Patient characteristics
| Characteristics | Number of Patients |
|---|---|
|
| |
| Median age (range), yr | 65 (34–78) |
|
| |
| Male sex, n(%) | 9 (45) |
|
| |
| Diagnosis, n(%) | |
| AML, induction | 19 (95) |
| AML, relapse | 1 (5) |
|
| |
| Chemotherapy, n(%) | |
| Cytarabine-containing regimen | 9 (45) |
| Clofarabine-containing regimen | 8 (40) |
| Miscellaneous* | 3 (20) |
AZD1152 (an experimental aurora kinase inhibitor) alone (n=1) or in combination with low-dose cytarabine (n=2)
Patients received prophylaxis for a median of 38 days (range: 5 to 42 days). Ten patients (50%) completed 42 days on posaconazole prophylaxis. Six patients (30%) had an early discontinuation of the study drug because of possible (1 patient) or probable (5 patients) IFI after a median of 13 days (range, 5 to 17 days) of therapy; one of these 6 patients also experienced intractable nausea and vomiting. In addition, one patient discontinued because of severe diarrhea, and 3 (15%) patients were unable to continue with oral medication due to odynophagia (n=1), brain hemorrhage (n=1) and respiratory failure (n=1).
Figure 1 shows the median levels of posaconazole for all patients. Three hours after the first dose, plasma levels reached a median of 124 ng/ml and increased to 220 ng/ml by 10 hours after the first dose. Baseline level by day 2 was 235ng/ml. There was evidence of drug accumulation up to 10 days from start of administration. By day 3, plasma levels after 10 hours were 435 ng/ml, and on day 10, 10 hours after administration, they reached 1000 ng/ml. Trough plasma levels on day 3 and 10 were 348 ng/ml and 895 ng/ml, respectively. Interestingly, plasma levels appeared to be higher for patients who completed therapy than for those who did not, with the difference increasing over time. On day 1, after 10 hours of drug administration, the medians plasma levels were 170 ng/ml for those who did not complete therapy and 290 ng/ml for those who did. By day 10, after 10 hours, plasma levels were 790 ng/ml and 1300 ng/ml, respectively.
Figure 1. Plasma concentrations for all patients after oral administration of posaconazole for antifungal prophylaxis.
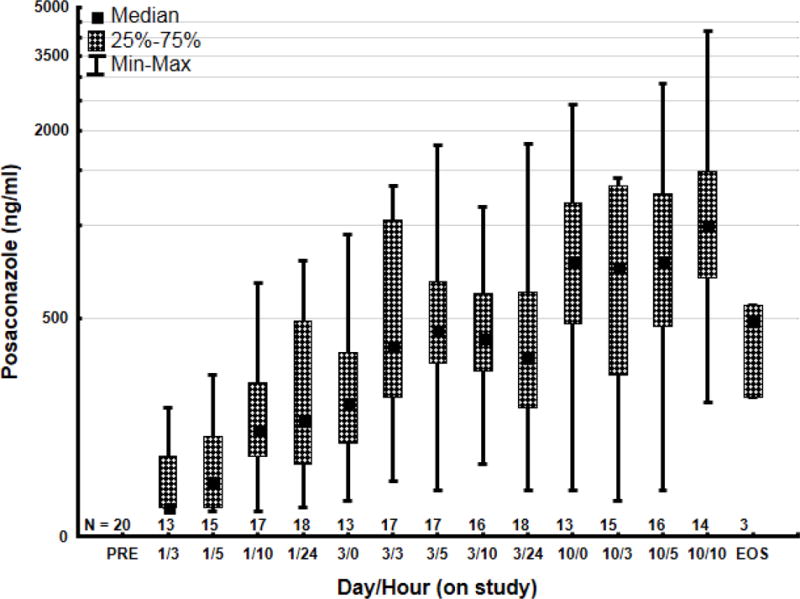
N= number of patients
Plasma levels were similar across different patient populations (Table 3). Trough plasma levels on day 4 were higher in male patients compared to female on day 4, but not on days 2 or 9 suggesting this difference is an artifact. Although there was a trend for higher trough plasma levels on day 2 and day 9 for patients with BSA <1.9, that did not reach statistical significance. A similar trend was observed for patients who did not develop an IFI who had a trend for higher plasma levels compared to those that experienced IFI. Similarly, there was no significant difference in plasma levels among patients who developed grade 3 or 4 elevation of liver enzymes and those without these events, although only 3 patients had a grade 3–4 elevation.
Table 3.
Posaconazole trough plasma levels by category
| Number | Median (Range) | Number | Median (Range) | p value | |
|---|---|---|---|---|---|
| Female | Male | ||||
| D2 H0 | 9 | 200 (140–660) | 9 | 290 (120–770) | 0.86 |
| D4 H0 | 10 | 300 (140–1820) | 8 | 580 (260–1090) | 0.04 |
| D10 H0 | 7 | 770(140–2450) | 6 | 800 (370–1690) | 0.84 |
| BSA<1.9m2 | BSA>1.9m2 | ||||
| D2 H0 | 8 | 380 (170–660) | 10 | 190 (120–770) | 0.27 |
| D4 H0 | 9 | 290 (140–1820) | 9 | 420 (250–1090) | 0.22 |
| D10 H0 | 7 | 810 (380–2450) | 6 | 500 (140– 1690) | 0.23 |
| No IFI | IFI | ||||
| D2 H0 | 13 | 350(140–770) | 5 | 170 (120–270) | 0.08 |
| D4 H0 | 14 | 410 (200–1820) | 4 | 310 (140–470) | 0.19 |
| D10 H0 | 11 | 810 (140–2450) | 2 | 640 (510–770) | 0.77 |
| No G3/4 LFT abnormalities | LFT abnormalities | ||||
| D2 H0 | 15 | 200 (140–770) | 3 | 270 (120–490) | 0.74 |
| D4 H0 | 16 | 410 (140–1820) | 2 | 270 (250–290) | 0.21 |
| D10 H0 | 11 | 540 (140–2450) | 2 | 980 (770–1180) | 0.64 |
Plasma posaconazole levels presented as ng/ml.
D, day; H, hour; BSA, body surface area; IFI, invasive fungal infection; G, grade; LFT, liver function test
4. Discussion
Fungal infections constitute a major cause of morbidity and mortality for patients with leukemia. In a recent report, nearly half of the 254 patients with newly diagnosed AML receiving intensive chemotherapy developed IFI [18]. In that series, antifungal prophylaxis was not routinely used. Anti-fungal prophylaxis is frequently used to decrease the risk of developing IFI and decreasing mortality [19]. Different modalities of antifungal prophylaxis have been investigated, including azoles, echinocandins and various formulations of amphotericin B with variable results [1–6, 18]. In a study of 602 patients with newly diagnosed or first-relapse AML or MDS receiving first induction chemotherapy, patients were randomized to receive prophylaxis with posaconazole (n=304) or either fluconazole (n=240) or itraconazole (n=58) [5]. Patients in the posaconazole group had a significantly lower incidence of proven or probable IFI (2%) compared to the control arm (8%). Fewer instances of invasive aspergillosis were also diagnosed in the posaconazole group compared to the control group (1% vs. 7%, respectively), resulting in improved survival [5]. A similar trial in recipients of stem cell transplant with severe graft-versus-host disease receiving immunosuppressive therapy, also showed superiority of posaconazole in reducing the risk of all IFI (5% vs. 9%) and invasive aspergillosis (2% vs. 7%).[6]
Posaconazole has adequate bioavailability but absorption is highly dependent on several factors, including diet. Administration of posaconazole in a fed state increases absorption, particularly when given with a high-fatty meal [8]. In a study of healthy volunteers, the bioavailability of an oral suspension of posaconazole increased 4-fold when administered with a high-fat diet, and 2.6-fold when administered with a low-fat diet [8]. Maintaining adequate diet in patients with AML receiving intensive chemotherapy is difficult because of the effects of the chemotherapy itself. In our study, nearly all patients referred presence of nausea and vomiting despite the use of antiemetic prophylaxis. Although we intended to keep a diary for food intake during the duration of the study, more than half of patients were unable to keep the diary accurately. This, together with the small sample size of this study prevented us from drawing any conclusions related to diet effect.
We demonstrated that the median posaconazole plasma levels were lower in patients who did not complete full course of prophylactic therapy. Overall, 10 patients had to discontinue posaconazole. Four of them had to stop therapy due to gastrointestinal side effects or poor swallowing. It is possible that these patients were not able to receive posaconazole properly due to mentioned clinical problems, and this led to lower plasma concentrations.
Another important factor in the pharmacokinetics of posaconazole administered orally is the frequency of administration. Studies in healthy volunteers have shown that when the total daily dose of 800mg is administered as two doses of 400mg, the bioavailability almost doubles compared to a once daily administration. Furthermore, administration as 4 doses of 200mg increases bioavailability approximately 3-fold [20]. With these split dosing schedules, steady state is observed on approximately day 10 after the start of therapy with no further accumulation after 14 days [21]. In our study, we administered posaconazole at a dose of 200mg three times daily. Our results show accumulation of posaconazole up to day 10, with trough plasma levels increasing from a mean of 332ng/ml on day 2 to 895ng/ml on day 10. Although peak concentrations have been reported to be achieved after 5 hours in healthy volunteers[21], our series suggests that peak concentrations are not achieved until later, at around 10 hours. Importantly, many of the studies previously reported have used tablet formulations rather than the oral suspension we used in our study. A study using three different schedules of an oral suspension among recipients of stem cell transplant showed Cmax on day one of 116–186 ng/ml, with steady state achieved at around 6 hours [22]. In our series, 5-hour plasma concentrations were similar to the series by Gubbins et al. However, peak concentrations were not achieved until later, with mean plasma levels at 10-hours of 275 ng/ml. Gubbins also reported that steady state had been achieved on study day 6, with mean trough levels of 192 ng/ml receiving posaconazole 200mg once daily, 219 ng/ml for those receiving 400mg once daily, and 414 ng/ml for patients receiving 200mg four times daily [22]. Using a dose schedule of 200 mg three times daily, we continue observing accumulation until day 10 with Cmin of 895 ng/ml. Taken together, our results suggest that the patient population we included in this analysis was able to achieve higher plasma concentrations of posaconazole using an oral suspension administered at a dose of 200 mg three times daily than what is reported in the literature in similar settings [23]. However, it is possible that higher steady state concentration in this study could be a variation resulting from small sample size of our study group and may not represent the general population.
In our series, we did not find a difference in exposure by sex or body surface area. Importantly, although we found no statistically significant difference in plasma concentrations between patients who eventually developed IFI and those without IFI, there was a trend for higher trough concentrations among patients with no IFI on day 2, 4 and 9 measurements. Trough plasma levels on day 2 were 2-fold higher (median 350 ng/ml) for those without IFI than those with IFI (170 ng/ml). Similar results were reported by Krishna et al among patients with stem cell transplant with GVHD, where Cmax was reported at 635 ng/ml among patients with IFI and 1360 for those without such infections[10].
These results suggest that pharmacokinetics of posaconazole vary from patient to patient. Patients with AML receiving induction chemotherapy who never develop IFI tend to have higher plasma concentrations after oral administration of posaconazole underscoring the importance of pharmacokinetics of this drug.
Table 2.
Outcome of prophylaxis
| Reason for discontinuation of study drug | Number of Patients (%) |
|---|---|
| Completed 42 days on study drug | 10 (50%) |
| Possible or probable IFI* | 6 (30%) |
| Severe diarrhea | 1 (5%) |
| Unable to continue with oral medication | 3 (15%) |
Includes one patient with concomitant untreatable nausea and vomiting
Acknowledgments
Funding: This Study was supported in part by Merck, and by MD Anderson Cancer Center’s Research Support Grant.
Footnotes
Competing Interests: J.C. received research support from Merck. Other authors have no relevant conflicts of interest to disclose.
References
- 1.Mattiuzzi GN, Alvarado G, Giles FJ, Ostrosky-Zeichner L, Cortes J, O’Brien S, et al. Open-label, randomized comparison of itraconazole versus caspofungin for prophylaxis in patients with hematologic malignancies. Antimicrob Agents Chemother. 2006;50:143–7. doi: 10.1128/AAC.50.1.143-147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattiuzzi GN, Estey E, Raad I, Giles F, Cortes J, Shen Y, et al. Liposomal amphotericin b versus the combination of fluconazole and itraconazole as prophylaxis for invasive fungal infections during induction chemotherapy for patients with acute myelogenous leukemia and myelodysplastic syndrome. Cancer. 2003;97:450–6. doi: 10.1002/cncr.11094. [DOI] [PubMed] [Google Scholar]
- 3.Mattiuzzi GN, Kantarjian H, Faderl S, Lim J, Kontoyiannis D, Thomas D, et al. Amphotericin b lipid complex as prophylaxis of invasive fungal infections in patients with acute myelogenous leukemia and myelodysplastic syndrome undergoing induction chemotherapy. Cancer. 2004;100:581–9. doi: 10.1002/cncr.11936. [DOI] [PubMed] [Google Scholar]
- 4.Mattiuzzi GN, Kantarjian H, O’Brien S, Kontoyiannis DP, Giles F, Zhou X, et al. Intravenous itraconazole for prophylaxis of systemic fungal infections in patients with acute myelogenous leukemia and high-risk myelodysplastic syndrome undergoing induction chemotherapy. Cancer. 2004;100:568–73. doi: 10.1002/cncr.11930. [DOI] [PubMed] [Google Scholar]
- 5.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. Fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–59. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 6.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–47. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 7.Lass-Florl C, Mayr A, Perkhofer S, Hinterberger G, Hausdorfer J, Speth C, et al. Activities of antifungal agents against yeasts and filamentous fungi: Assessment according to the methodology of the european committee on antimicrobial susceptibility testing. Antimicrob Agents Chemother. 2008;52:3637–41. doi: 10.1128/AAC.00662-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol. 2004;57:218–22. doi: 10.1046/j.1365-2125.2003.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walravens J, Brouwers J, Spriet I, Tack J, Annaert P, Augustijns P. Effect of ph and comedication on gastrointestinal absorption of posaconazole: Monitoring of intraluminal and plasma drug concentrations. Clin Pharmacokinet. 2011;50:725–34. doi: 10.2165/11592630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy. 2007;27:1627–36. doi: 10.1592/phco.27.12.1627. [DOI] [PubMed] [Google Scholar]
- 11.Krishna G, Moton A, Ma L, Medlock MM, McLeod J. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother. 2009;53:958–66. doi: 10.1128/AAC.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishel JJ, Sivik J. Breakthrough invasive fungal infection in an immunocompromised host while on posaconazole prophylaxis: An omission in patient counseling and follow-up. J Oncol Pharm Pract. 2008;14:189–93. doi: 10.1177/1078155208094123. [DOI] [PubMed] [Google Scholar]
- 13.Schlemmer F, Lagrange-Xelot M, Lacroix C, de La Tour R, Socie G, Molina JM. Breakthrough rhizopus infection on posaconazole prophylaxis following allogeneic stem cell transplantation. Bone Marrow Transplant. 2008;42:551–2. doi: 10.1038/bmt.2008.199. [DOI] [PubMed] [Google Scholar]
- 14.Ustun C, DeRemer DL, Steele JC, Jr, Forseen C, Fisher JF, Jillella AP. Fatal aspergillus fumigatus and candida glabrata infections with posaconazole prophylaxis after stem cell transplantation. Int J Antimicrob Agents. 2008;32:365–6. doi: 10.1016/j.ijantimicag.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: An international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 16.Wiederhold NP, Pennick GJ, Dorsey SA, Furmaga W, Lewis JS, 2nd, Patterson TF, Sutton DA, et al. A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations within the bloodstream and cerebral spinal fluid. Antimicrob Agents Chemother. 2014;58:424–31. doi: 10.1128/AAC.01558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson GR, 3rd, Rinaldi MG, Pennick G, Dorsey SA, Patterson TF, Lewis JS., 2nd Posaconazole therapeutic drug monitoring: A reference laboratory experience. Antimicrob Agents Chemother. 2009;53:2223–4. doi: 10.1128/AAC.00240-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neofytos D, Lu K, Hatfield-Seung A, Blackford A, Marr KA, Treadway S, et al. Epidemiology, outcomes, and risk factors of invasive fungal infections in adult patients with acute myelogenous leukemia after induction chemotherapy. Diagn Microbiol Infect Dis. 2012 doi: 10.1016/j.diagmicrobio.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruping MJ, Vehreschild JJ, Cornely OA. Primary antifungal prophylaxis in acute myeloblastic leukemia and myelodysplastic syndrome–still an open question? Leuk Lymphoma. 2010;51:20–6. doi: 10.3109/10428190903242602. [DOI] [PubMed] [Google Scholar]
- 20.Ezzet F, Wexler D, Courtney R, Krishna G, Lim J, Laughlin M. Oral bioavailability of posaconazole in fasted healthy subjects: Comparison between three regimens and basis for clinical dosage recommendations. Clin Pharmacokinet. 2005;44:211–20. doi: 10.2165/00003088-200544020-00006. [DOI] [PubMed] [Google Scholar]
- 21.Courtney R, Pai S, Laughlin M, Lim J, Batra V. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob Agents Chemother. 2003;47:2788–95. doi: 10.1128/AAC.47.9.2788-2795.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubbins PO, Krishna G, Sansone-Parsons A, Penzak SR, Dong L, Martinho M, et al. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob Agents Ch. 2006;50:1993–99. doi: 10.1128/AAC.00157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishna G, AbuTarif M, Xuan F, Martinho M, Angulo D, Cornely OA. Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy. 2008;28:1223–32. doi: 10.1592/phco.28.10.1223. [DOI] [PubMed] [Google Scholar]


