Abstract
Background
Neuropathic pain is a major pathology of the central nervous system associated with neuroinflammation. Ryk (receptor-like tyrosine kinase) receptors act as repulsive axon-guidance molecules during development of central nervous system and neural injury. Increasing evidence suggests the potential involvement of Wnt/Ryk (wingless and Int) signaling in the pathogenesis of neuropathic pain. However, its underlying mechanism remains unknown.
Results
The expression and location of Ryk receptor as well as its ligand Wnt1 were detected by qPCR, Western blot, and immunohistochemistry. We found that Ryk, a specific Wnt receptor, was expressed in IB4+ (Isolectin B4) and CGRP+ (calcitonin gene-related peptide) dorsal root ganglia neurons and their ascending unmyelinated fibers in the dorsal horn of the spinal cord. Ryk was upregulated after spinal nerve ligation surgery. Wnt1 was also increased in activated astrocytes in the dorsal horn after spinal nerve ligation. The presynaptic mechanism of Ryk in regulation of neuropathic pain was determined by electrophysiology in spinal slice. Spinal nerve ligation model was established, and the therapeutic potential of inhibiting Ryk receptor was determined. Spine-specific blocking of the Wnt/Ryk receptor signaling attenuated the spinal nerve ligation-induced mechanical allodynia but not thermal hyperalgesia. Further, it also blocked Ca2+-dependent signals including CaMKII and PKCγ, subsequent release of CCL2 (CCR-like protein) in the dorsal horn. An in vitro study showed that inactivating Ryk receptors with anti-Ryk antibodies or lentiviral Ryk shRNA led to the inactivation of Wnt1 for excitatory synaptic transmission in spinal slices and subsequent decrease in CCL2 expression in the dorsal root ganglia neurons.
Conclusion
These studies demonstrate the existence of critical crosstalk between astrocytes and unmyelinated fibers, which indicate the presynaptic mechanism of Ryk in cytokine transmission of neuropathic pain and the therapeutic potential for Wnt/Ryk signaling pathway in the treatment of neuropathic pain.
Keywords: Ryk receptor, Wnt, neuropathic pain, presynaptic regulation
Background
Neuropathic pain is a major pathology of the central nervous system (CNS) with no currently accepted treatment; therefore, it is difficult to treat after its onset. However, evidence suggests that local administration of anesthetics close to the time of injury can reduce the development of neuropathic pain.1 Chronic neuropathic pain is common among many patients following surgeries that involve intraoperative nerve damage, which may result from motor vehicle accidents, blasts and burns, and lower back injury.2–4 Intraoperative nerve damage can cause electrical hyperexcitability and the generation of abnormal nerve pulses in injured sensory neurons.5–7 The behavioral hallmark of this altered neuronal signaling is abnormal response to non-noxious stimuli and is termed allodynia. Therefore, to identify new therapeutic targets, a clear understanding of the molecular processes that are characteristically associated with neurotropic pain is of the utmost importance.
Wnts (wingless and Int), a typical family of glycoproteins, play a critical role in neural development.8–10 Emerging evidence indicates that Wnts may participate in disease progression in adult tissue,11–12 especially the spinal cord.13,14 Fernandez-Martos et al.15 have found that most Wnt ligands and inhibitors are expressed in the adult spinal cord of rats and are differentially induced via the Wnt/β-catenin signaling pathway, which is involved in glial scarring following spinal cord injury (SCI).15 Strategies seeking to modulate Wnt-dependent signaling pathways have shown to be beneficial in different experimental models of CNS disorders.16,17
Ryk (receptor-like tyrosine kinase) is a classical Wnt receptor16 with a Wnt inhibitory factor 1 (WIF1)-like extracellular domain that enables it to interact with different Wnt ligands, including Wnt1. Although the intracellular domain is catalytically inactive because of specific amino acid substitutions,18 the Ryk receptor is known to transduce extracellular signals across the plasma membrane through several mechanisms.19,20 Studies on CNS development have shown that Ryk receptors act as chemorepulsive axon-guidance molecules during the establishment of major axon tracts, such as in the corpus callosum and the corticospinal tract (CST).21,22 They are also vital for the generation of appropriate topographic maps of retinal ganglion-cell axons.23 Owing to the developmental role of Ryk as an essential regulator of axonal growth and the importance of this process in functional recovery following SCI, several studies have investigated the potential of this receptor to mediate axonal regeneration in experimental models of neurotropic pain.13,24 Blocking Ryk activity in corticospinal axons via intrathecal administration of a Ryk-neutralizing antibody resulted in a significant growth of axons in the CST and enhanced functional recovery following SCI.13,24 This strongly suggests that Ryk influences the progression of SCI, and modulating Ryk activity may improve chances of functional recovery. However, the influence of Ryk in SNL-mediated neurotrophic pain still remains unknown, which limits our understanding of its function in this neuropathological condition. Therefore, we sought to evaluate the spatiotemporal and cellular patterns of Ryk expression in the SNL model.
Methods
Animals
Male Sprague–Dawley rats (SD) (200–220 g) provided by Second Military Medical University, Shanghai, China, were used for these experiments. Rats were housed with ad libitum access to food and water, and under a 12:12-h light/dark cycle. The ambient temperature was maintained at 22 ± 0.6℃. All studies were approved by the Institutional Animal Care and Use Committee at Second Military Medical University and were in accordance with the guidelines of the International Association for the Study of Pain.
SNL model
The SNL procedure was performed as described previously.25 Briefly, animals were anesthetized with 4% chloral hydrate administered intraperitoneally (0.4 mL/100 g body weight). After separating the juxtaspinal muscles from the L3–S2 spinous processes, the right L6 transverse process was removed, and the left L5 spinal nerve was tightly ligated with a 6-0 silk thread. Sham-operated rats were treated with the same surgical procedure as the SNL rats, except that the left L5 spinal nerve was not ligated.
Preparation of spinal cord slice
Male 6- to 8-week-old SD rats were anaesthetized with intraperitoneal injection with urethane (1.5 g/kg). The spinal cord from L1–S3 was obtained and transported in a preoxygenated Krebs solution. The dura mater, ventral roots, dorsal roots, and the pia-arachnoid membrane of the spinal cord were removed. A 500-µm thick transverse slice of the spinal cord was cut at L3 or L4 using a microslicer. Then the slice was placed in the recording chamber and perfused with Krebs solution saturated at 95% O2 and 5% CO2 with a rate of 15–20 mL/min.
Patch-clamp recordings from in vivo preparations
Patch electrodes were pulled from thin-walled borosilicate glass capillaries (outside diameter 1.5 mm; World Precision Instruments, Sarasota, FL, USA) using a puller (p-97; Sutter Instrument, Novato, CA, USA). Patch electrodes were filled with a potassium gluconate-based internal solution (136 mM potassium gluconate, 5 mM KCl, 0.5 mM CaCl2, 2 mM MgCl2, 5 mM EGTA, 5 mM HEPES, and 5 mM ATP-Mg, pH 7.2). A patch electrode with a resistance of 8–12 MΩ was advanced at an angle of 30°–45° into the substantia gelatinosa (SG) neurons through the window using a micromanipulator (Model MP-1, Narishige). A Giga-ohm seal was formed with neurons at a depth of 30–150 µm from the surface of the spinal slice. This distance was identified to be within the SG neurons using transverse slices of the spinal cord at the same lumbar level (with bilateral SNL or without surgery). The membrane patch was ruptured by a negative pressure to form a whole cell configuration. A holding potential of −60 mV was used to record excitatory postsynaptic currents (EPSCs). Excitatory postsynaptic potentials were also recorded at resting membrane potentials. Data were stored on a personal computer using the pCLAMP data acquisition program (version 10.2, Axon Instruments) and analyzed using a software package (Mini Analysis, version 6.0.3; Synaptosoft Inc., Decator, GA, USA).
Implantation of the intrathecal catheter and drug delivery
For spinal drug delivery, all animals were implanted with an intrathecal catheter at the time of the SNL surgery. After excising the S1 processus spinalis, a polyethylene catheter (PE-10; 20.0 cm) was inserted into the subarachnoid space at the level of the L6–S1 vertebrae until the apex of the tube (1.5 cm) reached the lumbar cistern between the L4 and L5 vertebrae. The position of the catheters was fixed at the lumbar and the back of the head. Animals that exhibited motor dysfunction after an intrathecal injection of a small dose of lidocaine indicated successful implantation of the intrathecal catheter. The applied chemicals were limited to the L4–L5 DRG (dosal root ganglia) level by using a small volume (10 µl) of the injectant and limiting the rate of the injection (3.3 µl/min).26 The intrathecal application of drugs was carried out after one to three days of the SNL surgery. The Wnt inhibitor, IWP-2 (Stemgent), was dissolved in DMSO to final concentrations of 50 µM, 10 µM, and 1 µM. Anti-Ryk antibodies (Shanghai Institutes for Biological Sciences) were administrated at a concentration of 2 µg (dilution 1:10).27
Mechanical allodynia
On postoperative day 0, 3, 5, 7, and 14, animals were processed for behavioral tests. Paw-withdrawal threshold (PWT) was measured in response to mechanical stimulation using von Frey filaments.28,29 Rats were placed in a plastic chamber (20 × 20 × 25 cm3) for at least 30-min for behavioral adaptation. The plastic chamber was placed atop a mesh screen, allowing complete access to the paws from below. The von Frey filaments were applied to the ventral side of the left hind paw, between the third and fourth digits (nine calibrated von Frey hairs with bending forces of 0.4–15.0 g). If no withdrawal response (negative) were observed, a following higher force was delivered. Positive and negative responses were recorded and converted to a 50% threshold based on a formula provided by Chaplan.28 PWT measurements were reported in 10 min intervals over a 60-min observation period. If the 15 g failed to evoke a withdrawal response, the value is recorded as 15 g. A decreased response in paw withdrawal to a 7-g von Frey filament indicated mechanical hyperalgesia.
Thermal hyperalgesia
Heat hyperalgesia was detected using the paw withdrawal test.30 Rats were placed under an inverted clear plexiglass cage (20 × 18 × 13 cm3) on an elevated glass floor (3-mm thick). After adaption for 30 min, paw-withdrawal latencies (PWLs) to radiating heat were measured (automatic plantar analgesia tester, Institute of Biomedical Engineering, Chinese Academy of Medical Science, Tianjin, China) and used as the pain threshold. Temperature of the glass plate was adjusted to limit the baseline PWLs of normal rats to 6–10 s, and a cut-off time of 20 s was considered to avoid any tissue damage. Tests were performed three times for each paw, with a 5-min interval between each paw, and PWLS for all trials were averaged.
Preparation of rat DRG neurons and shRNA transfection
DRG from all levels were obtained from 10 adult male SD rats, and neuronal cultures were prepared as described previously.31 Cell cultures were incubated in BSF2 medium (containing 2% heat inactivated fetal calf serum, 0.1 mg/mL transferrin, 0.16 mg/mL sodium selenite, 3 mg/mL bovine serum albumen (BSA), penicillin/streptomycin 100 mg/mL each, 16 mg/mL putrescine, 10 mg/mL insulin) and NTFs for 24 h. DRG neurons were infected with Lentivirus-Ryk shRNA (GATCCCCGTCCAGGTTGAATATAAGTTCAAGAGACTTATATTCAACCTTGGACTTTTTGGAAA) or NC virus (TTCTCCGAACGTGTCACGT) (Han Biotechnology) for 12 h and then replaced with normal medium and harvested 72 h later.
q-PCR analysis
Total RNA from cells were isolated using an Ultraspec RNA isolation kit (Biotecx). Triplicate real-time PCR samples were obtained using Taqman probes and the ABI Prism 7000 sequence detection system. The primer sequences for target genes were selected (Supplementary Table 1). Samples were then quantified using the 2−ΔΔCT method, where CT is the cycle threshold. Data are expressed as 2−ΔΔCT. All ΔCt values were normalized to GAPDH.
Western blot analysis
For Western blot analysis, proteins separated by SDS-PAGE were electroblotted onto a PVDF membrane (Millipore, Billerica, MA). After blocking with 10% skimmed milk diluted in TBST, the membranes were incubated in primary antibodies overnight at 4℃ and incubated in horseradish peroxidase-conjugated secondary antibody (Kangcheng, Shanghai, China) the next day. After three washes, the blots were visualized using an ECL reaction system (Tannon).
Enzyme-linked immunosorbent assay and immunochemistry
Measurement of CCL2 (CCR-like protein) and BDNF in culture supernatant were determined by enzyme-linked immunosorbent assay (ELISA) with specific reagent kits (R&D Systems, Inc., USA) following the manufacturer’s instructions.
Then in vitro cultured cells were gently rinsed with PBS three times, fixed with 4% paraformaldehyde (pH 7.4) for 15 min at room temperature and permeabilized with 0.2% Triton X-100 for 5 min.
For immunohistochemistry, animals were anesthetized and perfused transcardially with 4% paraformaldehyde (pH 7.2). The spinal cord was removed and post-fixed for 4 h in the same fixative. After cryopreservation in 20% sucrose in 4℃, the tissue was cut into 14-µm thick frozen sections and incubated in 5% BSA containing 0.5% Triton X-100 for 20 min at room temperature. Cells or frozen sections were incubated with primary antibodies overnight at 4℃ and stained with FITC-conjugated or TRITC-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories and Vector Laboratories). Hoechst 33342 (1:1000; Sigma) was used to stain the nucleus. Images were obtained using a Nikon fluorescent microscope or a Leica SP5 confocal microscope.
Statistical analysis
Data were represented as mean ± SEM. The statistical significance of differences was analyzed using the PASW statistical program (SPSS Inc.). Statistical analyses were performed using a Student’s t test and one-way ANOVA. For behavioral responses, two-way ANOVA with repeated measure analyses of variance was performed followed by the Holm–Sidak post-hoc test for multiple comparisons. Differences were considered significant at p < 0.05.
Results
Increased expression of Ryk in DRG and spinal cord after SNL
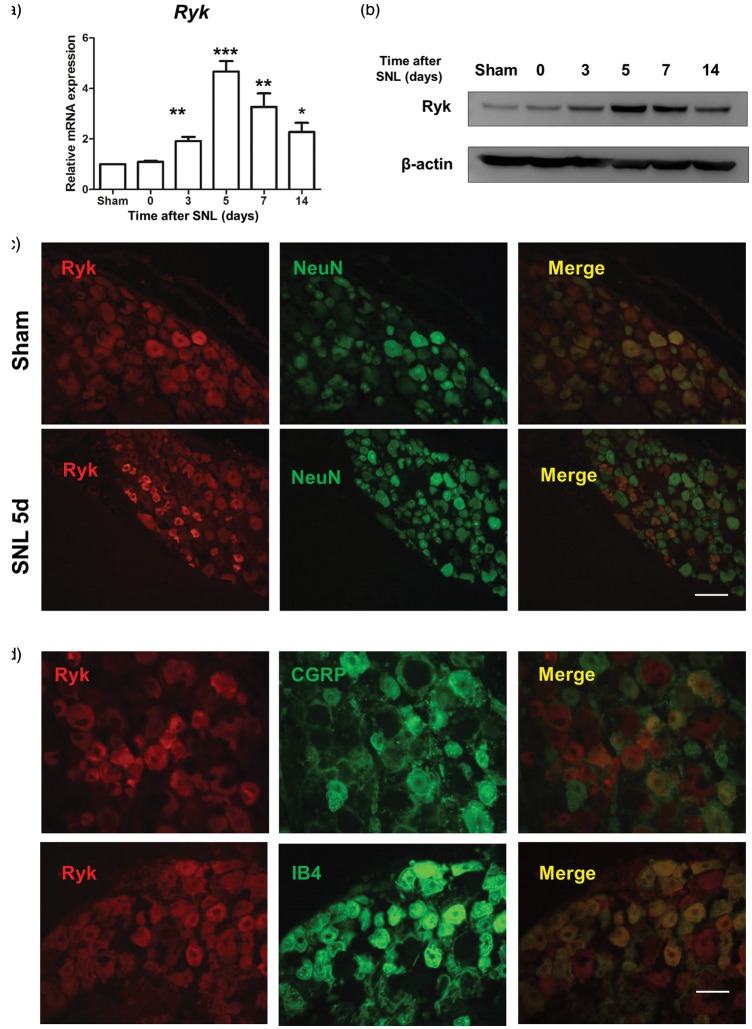
To characterize the effects of Ryk in DRG neurons after SNL surgery, we first used qPCR to examine Ryk expression in the lesion area. Results showed that from days 3–14 after the SNL surgery, Ryk mRNA levels were significantly higher in SNL rats than in sham-operated control rats, especially on post-SNL day 5 (Figure 1(a)). Similar results were seen on Western blot analysis. On post-SNL day 5, Ryk protein levels were significantly higher in SNL rats than in sham-operated control rats (Figure 1(b)).
Figure 1.
Ryk expression is increased in DRG neurons after SNL. (a) qPCR result showing the time course of Ryk mRNA expression in DRG lesions after SNL surgery. (b) Western blot showing the time course of Ryk protein expression in SNL lesions. (c) Distribution and cellular colocalization of Ryk expression (red) in the DRG with NeuN-IR (green). (Scale bar, 100 µm). (d) Distribution and cellular colocalization of Ryk expression (red) in DRG neurons with IB4 and CGRP (green). Data are shown as mean ± SEM (n = 4 in each group in a). *p < 0.05, **p < 0.01, ***p < 0.001 versus sham in the corresponding group (one-way analysis of variance). (Scale bar, 50 µm).
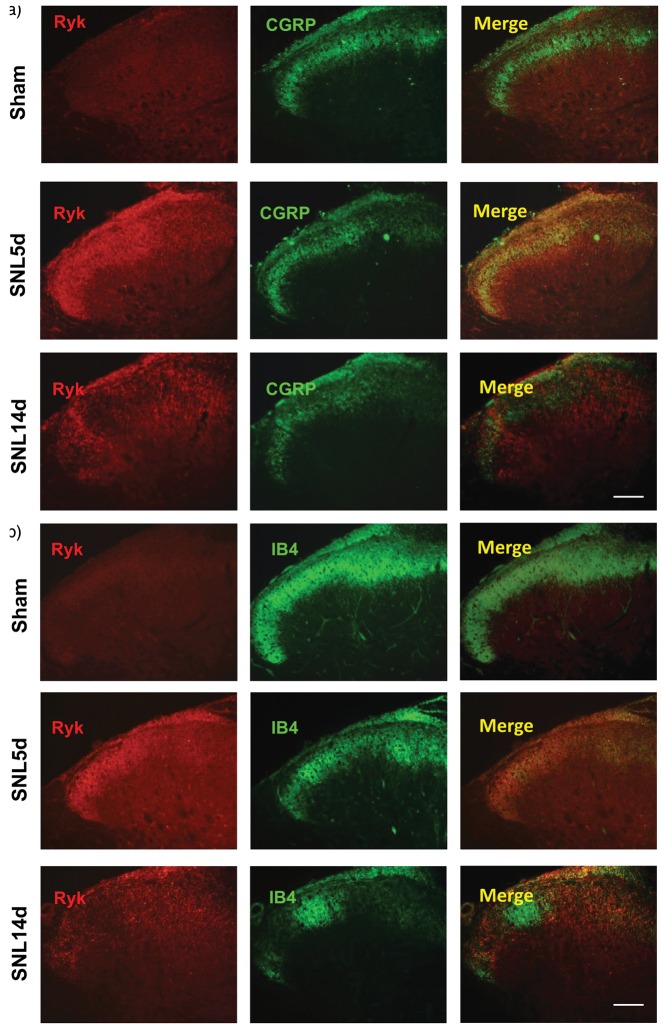
We performed immunohistochemistry to determine the location of Ryk receptors and NeuN was used as a marker for all neurons. We found that larger neurons were Ryk+ in DRG of sham-operated control rats; smaller neurons were Ryk+ in SNL rats on postoperative day 5. Next, immunohistochemistry was performed using IB4 (Isolectin B4) and CGRP (calcitonin gene-related peptide; markers for small diameter neurons) antibodies. Ryk receptors were found in IB4-labeled, and CGRP-labeled neurons, on postoperative day at five days in SNL rats (Figure 1(c) and (d)). As IB4 and CGRP positive neurons send unmyelinated nerve fibers into the dorsal horn of the spinal cord and regulate pain signaling, we further examined the change in expression of Ryk protein after SNL surgery by immunohistochemistry. The results showed a significant increase in Ryk protein expression in both injured CGRP-positive and IB4-positive fibers on postoperative day 5. However, expression levels decreased on postoperative day 14 (Figure 2(a) and (b)).
Figure 2.
Changes in location of Ryk expression in the dorsal horn after SNL. (a) Cellular colocalization of the Ryk receptor (red) in spinal cord after SNL injury with the unmyelinated fibers identified with IB4 (green) during the time course of SNL surgery in sham group, 5 days group and 14 days group. (Scale bar, 100 µm). (b) Cellular colocalization of the Ryk receptor (red) in spinal cord after SNL injury with the unmyelinated fibers identified with CGRP (green) during the time course of SNL surgery in sham group, 5 days group and 14 days group. (Scale bar, 100 µm).
Increased Wnt expression in activated astrocytes after SNL surgery
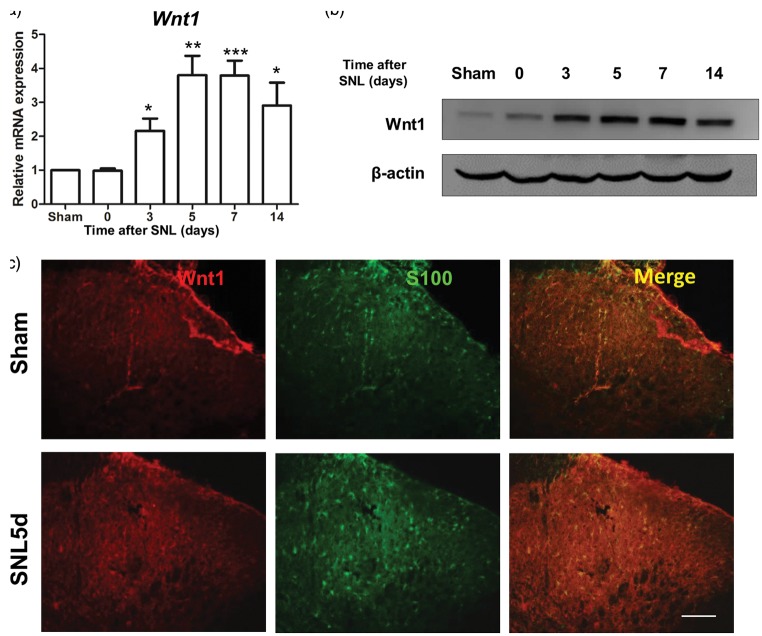
Expression levels of Wnt1, a classic ligand of Ryk, were also examined after the SNL surgery. Results showed that from post-SNL days 3–14, the levels of Wnt1 mRNA were significantly higher in SNL rats than in sham-operated control rats (Figure 3(a)). Wnt1 protein expression was detected by a Western blot analysis in SNL rats and sham-operated control rats. The results also showed that from postoperative days 3–14, Wnt1 protein levels were significantly higher in SNL rats than in sham-operated control rats (Figure 3(b)). Immunohistochemistry showed that Wnt1 was significantly expressed only in S100-positive (marker for activated astrocytes32) cells on postoperative day 5.
Figure 3.
Wnt1 expression increased in astrocytes in the dorsal horn after SNL. (a) qPCR result showing the time course of Wnt1 mRNA expression in DRG lesions after SNL. (b) Western blot showing the time course of Wnt1 protein expression in SNL lesions. (c) Distribution and cellular colocalization of Wnt1 expression (red) in the superficial dorsal horn with activated astrocytes (S100, green). Data are shown as mean ± SEM (n = 4 in each group in a). *p < 0.05, ***p < 0.001 versus sham in the corresponding group (one-way analysis of variance). (Scale bar, 100 µm).
Effects of Wnt1/Ryk signaling on excitatory synaptic transmission in SG neurons in spinal cord slices from control and SNL rats
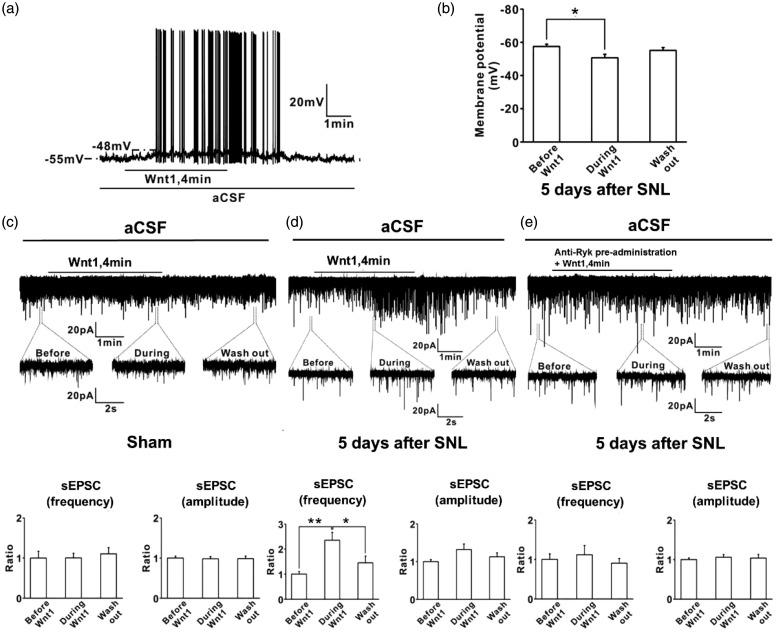
Neurons of the SG in the spinal dorsal horn play an important role in the transmission and modulation of nociceptive information from the periphery to the CNS.1 This is one of the key sites for synaptic plasticity (central sensitization) after nerve injury. Synaptic plasticity is partly exhibited as changes in spontaneous excitatory and inhibitory postsynaptic currents (sEPSCs and sIPSCs, respectively), which could indicate both a presynaptic mechanism (change in frequency) and a postsynaptic mechanism (change in amplitude).
First, we examined the passive membrane properties of SG neurons in the spinal cord slices from SNL rats. The average resting membrane potential was 57.5 ± 1.5 mV (n = 6). Application of Wnt1 (200 ng/ml, 4 min) caused membrane depolarization (increase of 6.8±1.6 mV) (p = 0.04, n = 6) and generated action potentials (Figure 4(a) and (b)). To determine whether activation of Ryk by Wnt1 affected excitatory neurotransmission within the spinal dorsal horn, we performed whole-cell patch-clamp recordings of sEPSCs in SG neurons in spinal cord slices of control and SNL rats. Membrane potential was voltage clamped at−70 mV. In the control group, bath application of Wnt1 (200 ng/ml, 4 min) had no effect on sEPSC frequency (100.5 ± 11.5% of the baseline control) (p = 1.0, n = 8) or amplitude (98.1 ± 5.6% of the baseline control) (p = 0.971, n = 8) (Figure 4(c)). However, bath application of Wnt1 (200 ng/ml, 4 min) strongly enhanced the frequency of sEPSCs (235.8 ± 30.6% of the baseline control) (p = 0.002, n = 13); however, it did not change the amplitude of sEPSCs significantly (132.0 ± 14.9% of the baseline control) (p = 0.119, n = 13) in the SG neurons of SNL rats (Figure 4(d)). The effect of Wnt1 on the frequency of sEPSCs was inhibited by pretreatment with anti-Ryk antibody in SNL rat spinal cord slices (p = 0.865, n = 13) (Figure 4(e)). These results indicated that acute local activation of Ryk by Wnt1 enhances excitatory neurotransmission in SG neurons of SNL rats.
Figure 4.
Wnt1/Ryk signaling enhanced excitatory synaptic transmission in SG neurons in spinal cord slices from control and SNL rats. (a, b) Passive membrane properties of SG neurons in spinal cord slices from SNL rats were detected with the administration of Wnt1. The average resting membrane potential was 57.5 ± 1.5 mV (n = 6). Application of Wnt1 (200 ng/ml, 4 min) caused membrane depolarization (increase of 6.8 ± 1.6 mV) (p = 0.04, n = 6) and generated action potentials. (c–e) Whole-cell patch-clamp recordings of sEPSCs in SG neurons in spinal cord slices of sham and SNL rats: (c) membrane potential was voltage clamped at −70 mV. In sham group, bath application of Wnt1 (200 ng/ml, 4 min) had no effect on sEPSC frequency (100.5 ± 11.5% of the baseline control) (p = 1.0, n = 8) or sEPSC amplitude (98.1 ± 5.6% of the baseline control) (p = 0.971, n = 8). (d) Bath application of Wnt1 (200 ng/ml, 4 min) strongly enhanced the frequency of sEPSCs (235.8 ± 30.6% of the baseline control) (p = 0.002, n = 13), whereas Wnt1 did not change the amplitude of sEPSCs significantly (132.0 ± 14.9% of the baseline control) (p = 0.119, n = 13) in SG neurons in SNL rats. (e) The effect of Wnt1 on the frequency of sEPSCs was inhibited by pretreatment with anti-Ryk in SNL rat spinal cord slices (p = 0.865, n = 13).
Wnt1 mediated CCL2 release via Ryk receptor
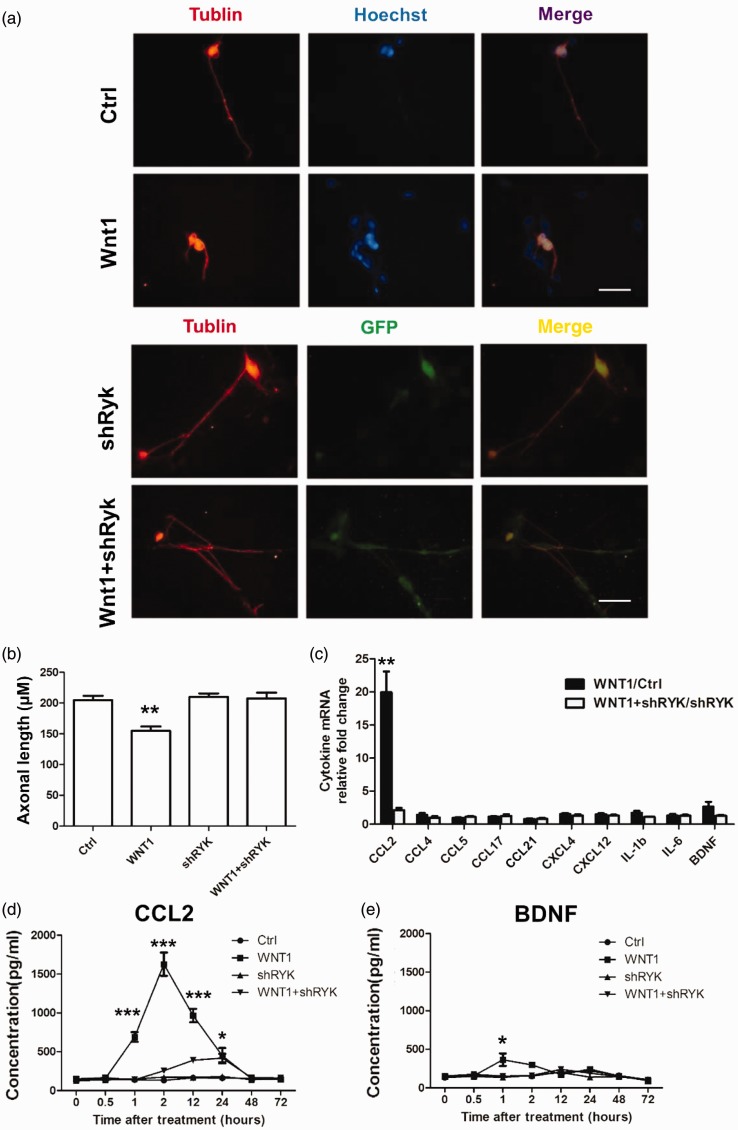
DRG neurons express Ryk receptors and may release neurotransmitters to unmyelinated fibers to modulate pain signals. To investigate the function of Wnt1/Ryk in neuropathic pain, an in vitro DRG culture was used (Figure 5(a)). DRG neurons were cultured separately, and 5 µM Wnt1 recombinant protein was added to the culture. Interestingly, a reduction in neural prominence was observed. Thus, we measured the axonal length (Figure 5(b)). When Ryk was downregulated by lentiviral-mediated shRNA interference, Wnt1 failed to reproduce this reduction in the DRG culture (Figure 5(a) and (b)). To determine whether this morphological change was associated with pain regulation, the level of mRNA of a series of inflammatory cytokines (CCL2, CCL4, CCL5, CCL17,CCL21, CXCL4, CXCL12, IL-1β, IL-6) and a growth factor (BDNF) were examined using qPCR in conditions with only Wnt1 stimulus, only lentiviral-mediated shRNA interference of Ryk or both. The results showed that the amount of CCL2 mRNA was upregulated after the Wnt1 stimulus; however, it was downregulated after shRNA of Ryk gene expression via lentivirus administration. ELISA indicated that CCL2 release increased predominantly after 1 h of Wnt1 addition in the culture medium; this increase lasted 23. However, this effect was attenuated in the Ryk shRNA group (Figure 5(c)). BNDF release was found to only increase slightly after 1 h of Wnt1 addition into the culture medium; this effect was also attenuated in Ryk shRNA group. These results indicated that Wnt1 may regulate CCL2 production and release via Ryk receptors to induce an inflammatory response.
Figure 5.
Wnt1 mediates CCL2 production and secretion from DRG neurons via Ryk receptors. (a) Morphological changes associated with administration of Wnt1 recombinant protein or lentiviral-mediated shRNA in primary culture of DRG neurons in vitro (Tublin, red and GFP, green). Knock down of Ryk with shRNA rescues the prominence regression effect induced by Wnt1. (Scale bar, 50 µm). (b) Statistical analysis of axon length of DRG neurons shown in (a). (c) qPCR analysis for expressional changes in of cytokine levels in cells treated with control, shRNA, Wnt1 with or without shRNA. Data are shown as mean ± SEM. **p < 0.01 Wnt1/Ctrl groups versus Wnt1 + shRNA/shRNA (Student’s t test). (d, e) ELISA analysis for CCL2 and BDNF in cells treated with control, shRNA, and Wnt1 with or without shRNA. Data are shown as mean ± SEM. **p < 0.05,**p < 0.01, ***p < 0.001, control versus corresponding group at the indicated times (one-way analysis of variance).
Wnt1 regulates SNL-induced mechanical allodynia via Ryk receptors
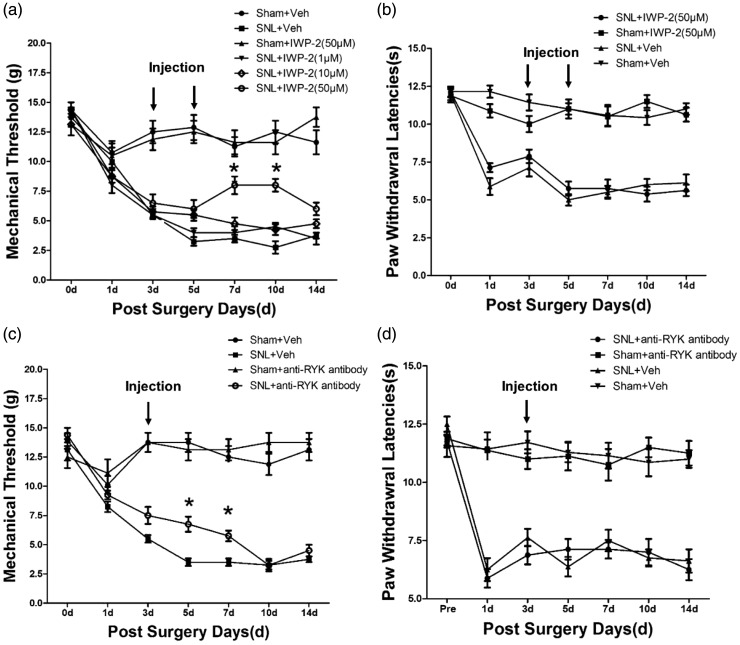
To verify whether Wnt/Ryk signaling regulates SNL-induced mechanical allodynia, IWP-2 (a Wnt inhibitor) was given to the rats through an intrathecal catheter within 1–14 days after SNL surgery. On postoperative days 1–3, the mean PWT were reduced dramatically below 6 g, confirming a successful model. The volume of IWP-2 was restricted to 10 µl, and administered to SNL rats at a moderate rate (3.3 µl/min). Next, rats were randomly divided into groups of eight each. Three groups received intrathecal injection of IWP-2 at graded doses of 1 µM, 10 µM, and 50 µM, respectively. Meanwhile, one sham group and one SNL group received equal volumes of vehicle injection as a control. Furthermore, we tested the PWT for mechanical pain after intrathecal injection of IWP-2. We found that the SNL-induced mechanical allodynia was significantly attenuated in the 50-µM group after 7–10 days of the SNL surgery; however, this effect did not last more than 14 days (Figure 6(a)). Additionally, vehicle or IWP-2 treatment in sham-operated control rats did not show any effect on mechanical allodynia. However, administration of IWP-2 after the SNL surgery did not affect the PWLs in the thermal hyperalgesia tests (Figure 6(b)).
Figure 6.
Wnt1/Ryk regulated SNL-induced mechanical allodynia but not heat hyperalgesia in vivo. (a and b) Mechanical allodynia and heat hyperalgesia were measured using the sham-operated rats with vehicle injection as the negative control (Sham + Veh, n = 8), SNL-injured rats with vehicle injection (SNL + Veh, n = 8), SNL-injured rats with graded doses (1µM, 10 µM, and 50 µM) of IWP-2 (SNL + IWP-2, n = 8), and sham-operated control rats with IWP-2 injection (Sham + IWP-2, n = 8, 50 µM) injection as the positive control. (c and d) Mechanical allodynia and heat hyperalgesia were measured using sham-operated control rats with vehicle injection as the negative control (Sham + Veh, n = 7), SNL-injured rats with vehicle injection (SNL + Veh, n = 10), SNL-injured rats with anti-Ryk injection (SNL + anti-Ryk, n = 8, 2µg), and sham-operated control rats with anti-Ryk injection (Sham + anti-Ryk, n = 8, 2µg) as the positive control. Data are shown as mean ± SEM. *p < 0.05, **p < 0.01, compared to SNL + Veh group (two-way ANOVA with repeated-measures ANOVA).
We also tested the possible analgesic effects of anti-Ryk antibody (anti-Ryk) that functionally blocks the Ryk receptor, on the induction and persistence of mechanical allodynia and thermal hyperalgesia in SNL rats. With the goal of testing Wnt signaling in DRG and in the spinal dorsal horn, the drug was delivered via intrathecal administration. Results showed that five to seven days after the administration of anti-Ryk post SNL surgery, PWT increased significantly. Meanwhile, blocking Ryk receptors in SNL rats did not affect PWLs in the thermal hyperalgesia tests (Figure 6(d)).
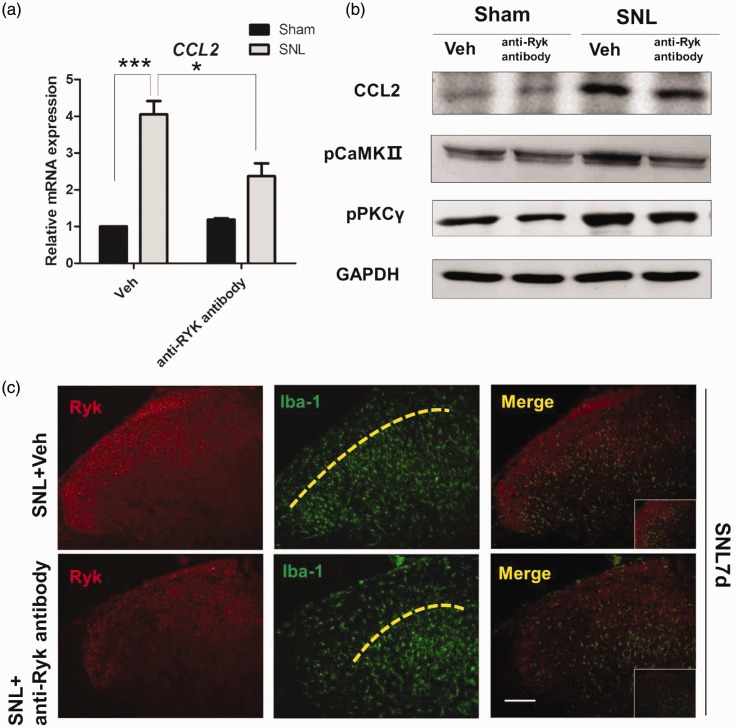
CCL2 is responsible for microglia activation.33 To analyze the inflammatory response of CCL2 after blocking Ryk receptors in SNL rats and sham-operated control rats, immunohistochemical staining for microglia in spinal cord slices showed that Ryk receptor highly expressed area could attract the microglia invasion. However, blocking Ryk receptors with anti-Ryk results in the reduction of microglia invasion into lamina II of the dorsal horn on postoperative day 7 (Figure 7(c)). Western blot analysis in SNL rats showed that CCL2 increased significantly in the dorsal horn, while treatment with anti-Ryk led to the reduction of CCL2. Simultaneously, downstream signaling of CCL2-induced inflammation was also detected. As cytokine release may be associated with Ca2+ signaling (also a pathway of Wnt signaling), phospho-CaMKII and phospho-PKCγ levels were tested. Both were increased within SNL rats; however, they were downregulated by the blocking effect of anti-Ryk (Figure 7(a) and (b)).
Figure 7.
Ryk activation after SNL surgery induced increased secretion of CCL2 through CaMKII/ PKCγ pathway. (a) Immunohistochemistry shows the expression of Ryk (red) and Iba-1(green) seven days after SNL surgery with and without the administration of anti-Ryk. (Scale bar, 100 µm). (b) Western blot analysis of CCL2, phospho-CaMKII, and phospho-PKCγ for proteins harvested from spinal cord seven days after surgery. Data are shown as mean ± SEM. *p < 0.05, ***p < 0.001 versus control in the corresponding group. (c) Immunohistochemical staining showed that in spinal cord with SNL surgery, Ryk (red) highly expressed area could attract microglia invasion (Iba-1, green), which is lamina II area of the dorsal horn. For microglia (Iba-1 positive) in spinal cord, blocking Ryk receptors with anti-Ryk results in the reduction of microglia invasion into lamina II area of the dorsal horn on post-operative day 7.
Discussion
In this study, we assessed the potential involvement of Wnt1/Ryk signaling in the underlying mechanism of neuropathic pain. Using the SNL model, we found that the ligation of the L5 nerve in rats upregulated the expressional level of Ryk receptors, while inhibiting Wnt1 and blocking Ryk receptors by neutralizing antibody gradually in accordance with the decline in the pain threshold, with diminished the function of Ryk.
Ryk is upregulated in DRG neurons and their fibers after SNL surgery, especially in the dorsal horn on postoperative day 5. This regulates pain induced by peripheral nerve injury. These results suggest that Ryk receptors are produced in DRG neurons and are transported, via axonal transport, to the spinal cord when peripheral nerves are injured. Meanwhile, Wnt1 RNA and protein levels are increased in activated astrocytes (labeled with S100 in the dorsal horn), three days after the SNL surgery. These results indicate that Wnt1 and Ryk levels correlate with SCI induced by the SNL surgery.
Electrophysiology analysis in spinal cord slices shows that Wnt1 regulates the membrane potential of SG neurons. Wnt1 administration in vitro caused membrane depolarization and generated action potentials. Wnt1 also enhanced the frequency of sEPSCs, which is a hallmark of central sensitization. However, preadministration of anti-Ryk to the culture inhibited this effect, which indicated that Ryk receptors play a key role in regulating pain. The enhancement of sEPSCs may be induced by increasing level of neurotransmitters or cytokines. In the in vitro studies, Wnt1 increased the production and release of CCL2, which was reduced by interfering with the expression of Ryk receptors. Our in vivo studies also identified that blocking Ryk receptors downregulated the expression of CCL2 in the dorsal horn. These observations suggested that CCL2 could be a downstream effector target in spinal Wnt1/Ryk signaling. CCL2 was reported to influence central sensitization induced by glial cells.33 CCL2 increases after nerve injury in DRG and spinal cord. It binds with high affinity to the chemokine receptor CCR2, a G-protein coupled receptor. Several cells, including microglia, express CCR2.33 Therefore, CCL2 could activate microglia and in turn, microglia may release a number of mediators that break the normal neural loop and increase the excitement of SG neurons, where the central sensitization was first initiated. These results indicate that during SNL surgery, upregulated Wnt1 secreted by activated astrocytes together with increased Ryk receptors on unmyelinated fibers promoted the production and release of CCL2. However, the process of CCL2 secretion may be controlled by Ryk receptors, since we found that phospho-CaMKII and phospho-PKCγ were activated by the SNL surgery and downregulated after blocking Ryk. These findings may support a new mechanism underlying neuropathic pain, thereby indicating a new therapeutic opportunity for its treatment.
In our study, blocking Ryk function attenuated inflammatory responses and decreased mechanical threshold but had no effect on thermal hyperalgesia. It is difficult to illustrate such phenomena. Mechanical allodynia and thermal hyperalgesia are two different characteristics of neuropathic pain which was controlled by two different kinds of unmyelinated fibers. In our study, we found the expression of Ryk and its change is different between these two fibers. Even though Ryk receptor was upregulated and colocalized with IB4 and CGRP in 7 days after surgery, it does not sustained in 14 days in CGRP positive fiber unlike IB4 positive fiber. Besides, thermal hyperalgesia was occurred earlier than mechanical allodynia in our model. This morphological difference may indicate that Ryk receptor may play distinct roles between thermal hyperalgesia and mechanical allodynia. In order to test the analgesic effect of blocking Ryk receptor, intrathecal injection of blocking drugs or antibody during days 3–5 after SNL were proceeded. However, before this time, thermal hyperalgesia was formed one day after SNL. So it is suggested that interfering Ryk receptors cannot block thermal pain may be associated with fiber feature or blocking time.
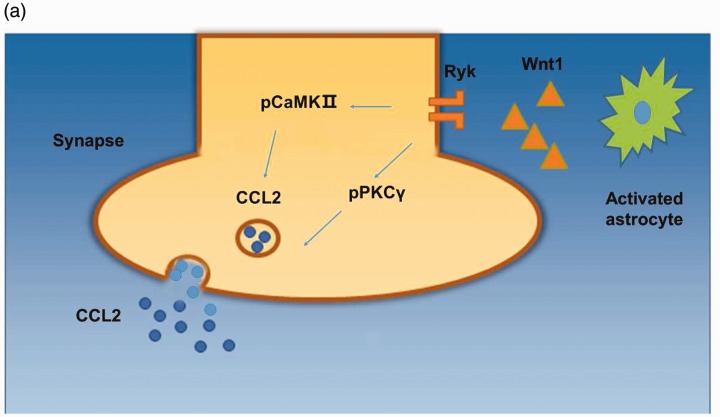
Hyperexcitability of SG neurons is one of the essential causes for central sensitization in the spinal cord, leading to neuropathic pain.27 Wnt proteins are important for various developmental processes. Studies have demonstrated the dysregulation of Wnt signaling in certain diseases and disorders.15 Wnt proteins have been demonstrated to regulate the development of neuropathic pain through the canonical, β-catenin signaling pathway.34 However, in this study, we found that Ryk receptors were mainly expressed in unmyelinated fibers, not in SG neruons, and activating Ryk receptors by astrocyte-derived Wnt1 increased CCL2 release, resulting in neurotrophic pain (Figure 8(a)). We conclude that Wnt/Ryk signaling plays a key role in glial-neural communication, which may be attributed to activation of inflammatory cells and central sensitization, providing an innovational target for the treatment of pain from SCI. Ryk, a transmembrane receptor, interacts with Wnt proteins through its extracellular WIF domain, which brings extracellular signals into the nucleus20,35,36 and activates phospho-CaMKII and phospho-PKCγ (Figure 8(a)). Blocking Ryk function with anti-Ryk significantly suppresses CaMKII and PKCγ activity, which mediate the release of CCL2, thereby relieving neurotrophic pain and restricting inflammatory response.
Figure 8.
Mechanism of Ryk activation in dorsal horn after SNL. (a) Wnt1 derived from activated astrocytes increased the expression and release of CCL2 by activating Ryk receptors and downstream Ca2+-dependent signals including CaMKII and PKCγ.
Conclusion
In this study, we demonstrated for the first time that Ryk, a specific Wnt receptor, was expressed in IB4+ and CGRP+ DRG neurons and their ascending unmyelinated fibers in the dorsal horn of the spinal cord. The expression of Ryk was upregulated after SNL surgery. Meanwhile, Wnt1 was also increased in activated astrocytes in the dorsal horn after SNL. Spine-specific blocking of the Wnt/Ryk receptor signaling attenuated the SNL-induced mechanical allodynia but not thermal hyperalgesia. Furthermore, it also blocked Ca2+-dependent signals including CaMKII and PKCγ, subsequent release of CCL2 in the dorsal horn. We showed the existence of critical crosstalk between astrocytes and unmyelinated fibers, which indicate the presynaptic mechanism of Ryk in cytokine transmission of neuropathic pain.
Authors’ Contributions
OYQ performed all experiments, analyzed the data, and drafted the manuscript. YWJ and LJ participated in the project planning and all Western blot and immunofluorescence experiments. LFT and OYQ participated in animal experiments. OYYP conceived of the project, coordinated and supervised the experiments, and revised the manuscript. YHB provide technical and administrative support. All authors read and approved the final manuscript. QOY, YWJ, and JL contributed equally to this work.
Supplementary Material
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval
All procedures were performed based on the guidelines established by the China Regulations for the Administration of Affairs Concerning Experimental Animals (1988), as well as the Committee for Protection, Supervision, and Control of Experiments on Animals guidelines of Secondary Military Medical University.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the projects from National Natural Science Foundation of China (Nos. 81171054, 81371253, and 81271236) and Innovation Program of Shanghai Municipal Education commission 14ZZ083.
References
- 1.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 2.Mercer SJ, Chavan S, Tong JL, et al. The early detection and management of neuropathic pain following combat injury. J R Army Med Corps 2009; 155: 94–98. [DOI] [PubMed] [Google Scholar]
- 3.Clark ME, Bair MJ, Buckenmaier CR, et al. Pain and combat injuries in soldiers returning from Operations Enduring Freedom and Iraqi Freedom: implications for research and practice. J Rehab Res Dev 2007; 44: 179–194. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SP, Griffith S, Larkin TM, et al. Presentation, diagnoses, mechanisms of injury, and treatment of soldiers injured in Operation Iraqi Freedom: an epidemiological study conducted at two military pain management centers. Anesth Analg 2005; 101: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 5.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012; 73: 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000; 288: 1765–1769. [DOI] [PubMed] [Google Scholar]
- 7.Wall PD, Waxman S, Basbaum AI. Ongoing activity in peripheral nerve: injury discharge. Exp Neurol 1974; 45: 576–589. [DOI] [PubMed] [Google Scholar]
- 8.Freese JL, Pino D, Pleasure SJ. Wnt signaling in development and disease. Neurobiol Dis 2010; 38: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781–810. [DOI] [PubMed] [Google Scholar]
- 10.Michaelidis TM, Lie DC. Wnt signaling and neural stem cells: caught in the Wnt web. Cell Tissue Res 2008; 331: 193–210. [DOI] [PubMed] [Google Scholar]
- 11.De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene 2006; 25: 7545–7553. [DOI] [PubMed] [Google Scholar]
- 12.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 2010; 11: 77–86. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Wang X, Lu CC, et al. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci 2008; 28: 8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan S, Shi Y, Tang SJ. Wnt signaling in the pathogenesis of multiple sclerosis-associated chronic pain. J Neuroimmune Pharmacol 2012; 7: 904–913. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, et al. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLOS ONE 2011; 6: e27000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mastroiacovo F, Busceti CL, Biagioni F, et al. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J Cereb Blood Flow Metab 2009; 29: 264–276. [DOI] [PubMed] [Google Scholar]
- 17.Parish CL, Castelo-Branco G, Rawal N, et al. Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J Clin Invest 2008; 118: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fradkin LG, Dura JM, Noordermeer JN. Ryks: new partners for Wnts in the developing and regenerating nervous system. Trends Neurosci 2010; 33: 84–92. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Hutchins BI, Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J Neurosci 2009; 29: 5873–5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W, Yamamoto V, Ortega B, et al. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 2004; 119: 97–108. [DOI] [PubMed] [Google Scholar]
- 21.Keeble TR, Halford MM, Seaman C, et al. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci 2006; 26: 5840–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Shi J, Lu CC, et al. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci 2005; 8: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt AM, Shi J, Wolf AM, et al. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature 2006; 439: 31–37. [DOI] [PubMed] [Google Scholar]
- 24.Miyashita T, Koda M, Kitajo K, et al. Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma 2009; 26: 955–964. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Li X, Jiang X, et al. Microvesicles shed from microglia activated by the P2X7-p38 pathway are involved in neuropathic pain induced by spinal nerve ligation in rats. Purinergic Signal 2017; 13: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Zhang X, Wang C, et al. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci USA 2008; 105: 16773–16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, Liu YP, Huang ZJ, et al. Wnt/Ryk signaling contributes to neuropathic pain by regulating sensory neuron excitability and spinal synaptic plasticity in rats. Pain 2015; 156: 2572–2584. [DOI] [PubMed] [Google Scholar]
- 28.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 29.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980; 20: 441–462. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Luo C, Li H, et al. Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: a comparative study with the formalin test. Pain 1999; 83: 67–76. [DOI] [PubMed] [Google Scholar]
- 31.Anand U, Otto WR, Sanchez-Herrera D, et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain 2008; 138: 667–680. [DOI] [PubMed] [Google Scholar]
- 32.Mrak RE, Griffinbc WS. The role of activated astrocytes and of the neurotrophic cytokine S100B in the pathogenesis of Alzheimer's disease. Neurobiol Aging 2001; 22: 915–922. [DOI] [PubMed] [Google Scholar]
- 33.Joachim S, Clifford JW. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007; 10: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YK, Huang ZJ, Liu S, et al. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest 2013; 123: 2268–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue T, Oz HS, Wiland D, et al. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell 2004; 118: 795–806. [DOI] [PubMed] [Google Scholar]
- 36.Lyu J, Yamamoto V, Lu W. Cleavage of the Wnt receptor Ryk regulates neuronal differentiation during cortical neurogenesis. Dev Cell 2008; 15: 773–780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.