Figure 2.
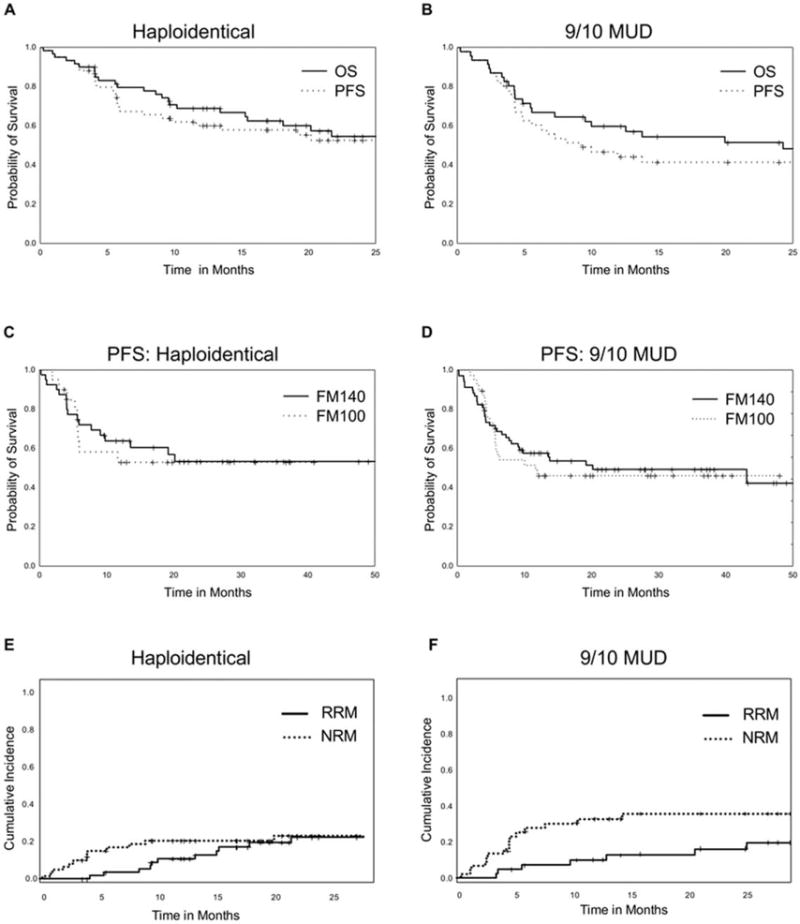
(A,B) Overall survival (OS) and progression-free survival (PFS) outcomes are illustrated in (A) the haploidentical donor arm and (B) the 1-antigen human leukocyte antigen-mismatched unrelated donor (9/10 MUD) arm. (C,D) PFS is illustrated according to conditioning intensity for those who received FM140 (1 intravenous dose of 140 mg/m2 melphalan on day −7, 1 intravenous dose of 5 mg/kg thiotepa on day −6, and 4 intravenous doses of 40 mg/m2 fludarabine [1 daily on days −5 through −2]) versus those who received FM100 (the same regimen with a lower melphalan dose of 100 mg/m2) in (C) the haploidentical donor arm and (D) the 9/10 MUD arm. (E,F) Relapse-related mortality (RRM) and nonrelapse-related mortality (NRM) are illustrated in (E) the haploidentical donor arm and (F) the 9/10 MUD arm.
