Abstract
Luminal A breast cancer has a much better prognosis than other subtypes, with a low risk of local or regional recurrence. However, there is controversy around under- versus overtreatment with regard to adjuvant treatment of node-positive, luminal A breast cancer. The purpose of this study was to identify whether adjuvant systemic chemotherapy has any benefit in node-positive, luminal A breast cancer and to evaluate feasibility of endocrine therapy without chemotherapy in this group.
This was a retrospective study of 11,025 patients who were surgically treated for invasive breast cancer at Samsung Medical Center between January 2004 and December 2013. Luminal A subtype was defined as ER+, HER2-, and Ki-67 < 14%. We compared AC based (AC: doxorubicin or epirubicin, plus cyclophosphamide) adjuvant chemotherapy versus endocrine therapy without chemotherapy in patients with node-positive, luminal A breast cancer.
We performed 1: n matching, with a maximum n of 8 on endocrine therapy group (n = 50) to chemotherapy group (n = 642). The median age of the patients in each group at the time of surgery was 58.3 ± 9.5 years in the chemotherapy group and 58.7 ± 11.7 in the endocrine therapy only group. The median follow-up time was 51.9 months (range, 1–125 months). In multivariable analysis, omission of adjuvant chemotherapy in luminal A cancer had no influence on OS and DFS. Axillary lymph node metastasis and progesterone receptor (PR) status were significantly different between the endocrine therapy alone group and the chemotherapy group in terms of OS. Nuclear grade, PR status, and adjuvant radiotherapy were significantly different between the endocrine therapy alone group and the chemotherapy group with regard to DFS. In survival analysis, there were no differences in OS (P = .137) and DFS (P = .225) between the 2 groups.
Adjuvant chemotherapy could provide little benefit to postmenopausal patients with luminal A, node-positive breast cancer, and endocrine therapy alone may help reduce morbidity. Future studies with a large number of patients and longer follow-up time are necessary to determine whether chemotherapy might be avoided in this patient population.
Keywords: adjuvant endocrine therapy, luminal A breast cancer, node-positive breast cancer, Prognosis
1. Introduction
Luminal A breast cancer is a common subtype defined by high expression of estrogen and progesterone receptors, and low expression of the cell-growth marker Ki-67 and the HER2 (human epidermal growth factor receptor 2) oncoprotein on a 6-marker immunohistochemical panel.[1] Evidence suggests that luminal A subtype breast cancer has a much better prognosis than other subtypes, with a low risk of local or regional recurrence. However, there is controversy around under- versus overtreatment with regard to adjuvant treatment of hormone receptor-positive (HR+) breast cancer.[2]
Although there is ongoing debate, adjuvant chemotherapy is widely used in the treatment of ER- and/or PR-positive breast cancer. In recent meta-analysis, additional adjuvant chemotherapy to tamoxifen had benefit in hormone receptor-positive breast cancer reducing the risk of recurrence and mortality by 30% and 20%.[3] Combination chemotherapy generally provides higher rates of objective response and longer time to progression in comparison to single agent chemotherapy. However, it is associated with an increase in toxicity and is of little survival benefit.[4–6] Current guidelines recommend the addition of adjuvant chemotherapy to hormonal therapy for patients with ER-positive, node-positive early breast cancer (EBC).[7] Many of these patients may remain disease-free even if they do not receive chemotherapy.[8] Thus, a proportion of patients with node-positive early breast cancer may be over treated, increasing healthcare costs and exposing patients to toxic adverse events related to chemotherapy with little additional benefit.
The purpose of this study was to identify whether adjuvant systemic chemotherapy has any benefit in node-positive, luminal A breast cancer and to evaluate feasibility of endocrine therapy without chemotherapy in this group.
2. Patients and methods
This was a retrospective study of 11,025 patients who were surgically treated for invasive breast cancer at Samsung Medical Center between January 2004 and December 2013. Patients were eligible if they met the following criteria: (1) luminal A breast cancer, (2) positive axillary lymph node status, and (3) radical excision of the primary tumor and axillary lymph node dissection (ALND). Luminal A subtype was defined as ER+, HER2-, and Ki-67 <14%. The hormone receptor status was determined using an enzyme immunoassay and reported in the medical record between January 2004 and December 2013. Immunohistochemistry (IHC), fluorescence in situ hybridization (FISH) or silver in situ hybridization (SISH), was performed to evaluate HER2 status, and an IHC score of +3 or FISH/SISH positivity was defined as positive for HER2 overexpression. Patients with bilateral breast cancer, inflammatory breast cancer, or distant metastasis were excluded. Patients treated with neoadjuvant chemotherapy and patients who had a previous or concurrent second malignancy were excluded.
We found 50 patients who had not received chemotherapy who were administered tamoxifen or an aromatase inhibitor, whereas the chemotherapy group received endocrine therapy plus AC-based (AC: doxorubicin plus cyclophosphamide) adjuvant chemotherapy (AC followed by docetaxel; AC followed by weekly paclitaxel; CAF (cyclophosphamide, doxorubicin, 5-fluorouracil) followed by taxanes (T)). In some patients, gonadotropin-releasing hormone (GnRH) agonist (3.6 mg) every 28 days for 2 years was used for ovarian function suppression. Overall survival (OS) was the primary endpoint. We also evaluated the disease-free survival (DFS) and the interval without distant metastasis.
2.1. Statistical analysis
To minimize selection bias and describe the treatment effects of different modalities in our observational study, we matched patients who did not receive adjuvant chemotherapy to those treated with chemotherapy at a ± 0.05 propensity score range.[9] We derived the propensity score from a logistic regression model using variables associated with the indication of chemotherapy (age, type of surgery, histological type, lymphovascular invasion, pathologic tumor size, pathologic nodal status, hormone receptor status, and use of GnRH agonist) to achieve the maximal group similarity for these parameters rather than on the basis of statistical significance. Thus, each patient was assigned a propensity score corresponding to the likelihood of receiving chemotherapy. We performed 1:n matching, with a maximum n of 8, on their propensity score ±0.05 using the “nearest-neighbor” matching method. We compared the survival of the group treated with chemotherapy with that of the endocrine therapy group (matched using propensity scores). To match participants, we used an automated matching procedure in SAS software version 9.4 (SAS Institute, Cary, NC) that randomly selected a treated individual and an untreated individual (comparator) from the pool of potential comparators to determine whether the patients fulfilled the matching criteria.
Chi-square or Fisher's exact tests were used to compare discrete variables. In a univariate analysis, overall survival (OS) and disease-free survival (DFS) were assessed using the Kaplan–Meier method, and log-rank tests were used to compare the differences between the resulting curves. Hazard ratios, 95% confidence intervals (CI) and multivariate survival analyses were performed using the Cox proportional-hazards model. SAS version 9.4 (SAS Institute, Cary, NC) was used for the chi-square test, Cox's proportional hazard regression, and logistic regression.
The study was approved by the institutional review board of the Samsung Medical Center, Seoul, Korea (approval numbers: 2016–04–056).
3. Results
Among 11,025 patients, we found 1267 patients with ER+, HER2-, Ki-67<14% and node-positive breast cancer; unknown HER2, Ki-67 status and chemotherapy other than AC based (AC: doxorubicin plus cyclophosphamide) group (AC followed by docetaxel; AC followed by weekly paclitaxel; CAF [cyclophosphamide, doxorubicin, 5-fluorouracil], followed by taxanes) were excluded. Of 870 women with node-positive luminal A breast cancer, a subsample was created of 692 women with overlapping propensity scores to receive chemotherapy. Propensity score matching improved the similarity in each factor distribution and resulted in overall propensity scores that were not significantly different after matching (Table 1). We performed 1:n matching, with a maximum n of 8 on the endocrine therapy group (n = 50) to the chemotherapy group (n = 642). After matching, there was no statistically significant difference in age, type of surgery, histologic grade, lymphovascular invasion, pathologic tumor stage, and node stage between the 2 groups. The median age of the patients in each group at the time of surgery was 58.3 ± 9.5 years in the chemotherapy group and 58.7 ± 11.7 in the endocrine therapy only group. The median follow-up time was 51.9 months (range 1–125 months). Among 50 patients with endocrine therapy alone, 17 patients refused chemotherapy, 4 patients did not undergo chemotherapy because of the underlying liver disease, and 5 patients did not undergo chemotherapy because of old age. Chemotherapy was considered of no benefit to 3 patients because of small tumor size and 20 patients did not undergo chemotherapy because of micrometastasis of lymph nodes.
Table 1.
Characteristics of chemotherapy ± endocrine therapy and endocrine therapy alone groups.
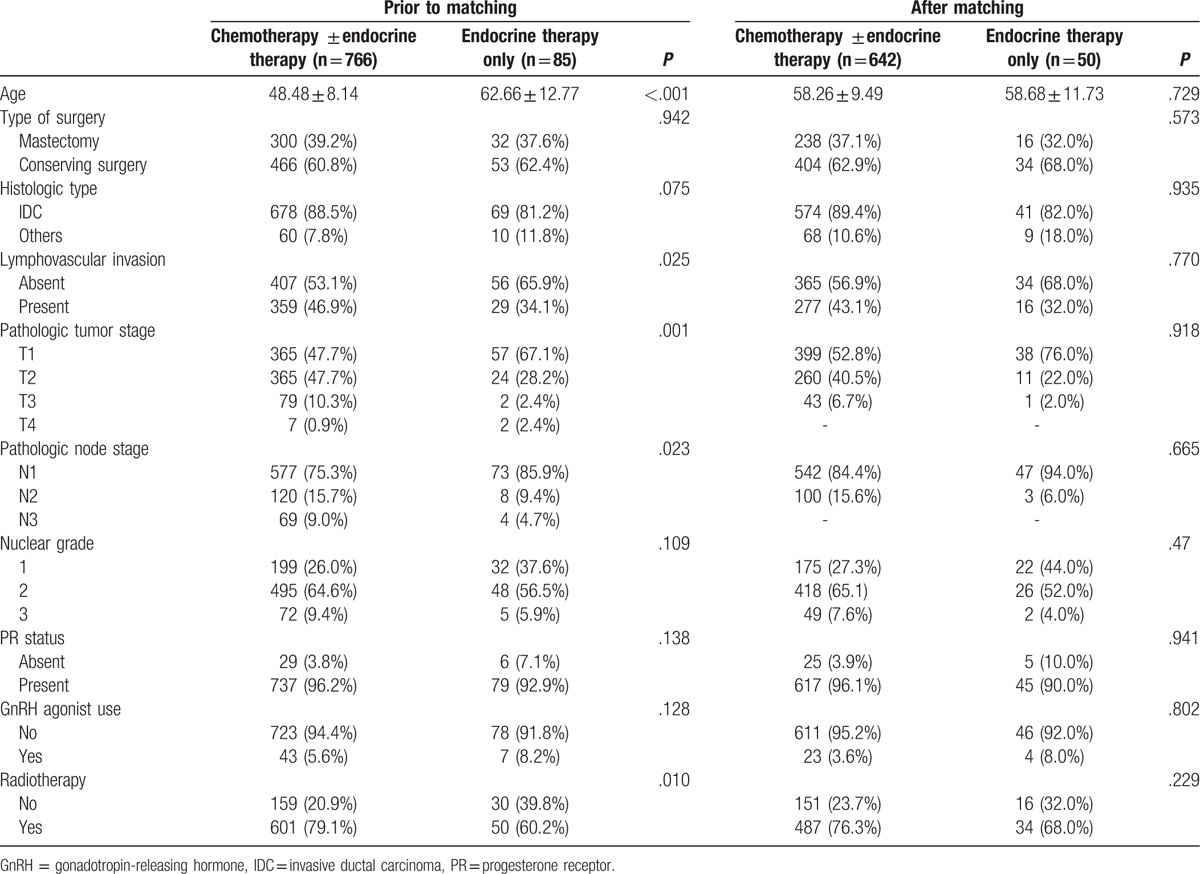
In multivariable analysis, omission of adjuvant chemotherapy in luminal A cancer had no influence on OS and DFS. Axillary lymph node metastasis and PR status were significantly different between the endocrine therapy alone group and the chemotherapy group in terms of OS (Table 2). Nuclear grade, PR status and adjuvant radiotherapy were significantly different between the endocrine therapy alone group and the chemotherapy group with regard to DFS (Table 3). In survival analysis, there were no differences in OS (P = .137) and DFS (P = .225) between the 2 groups (Fig. 1).
Table 2.
Univariate and multivariate Cox regression analyses for overall survival.
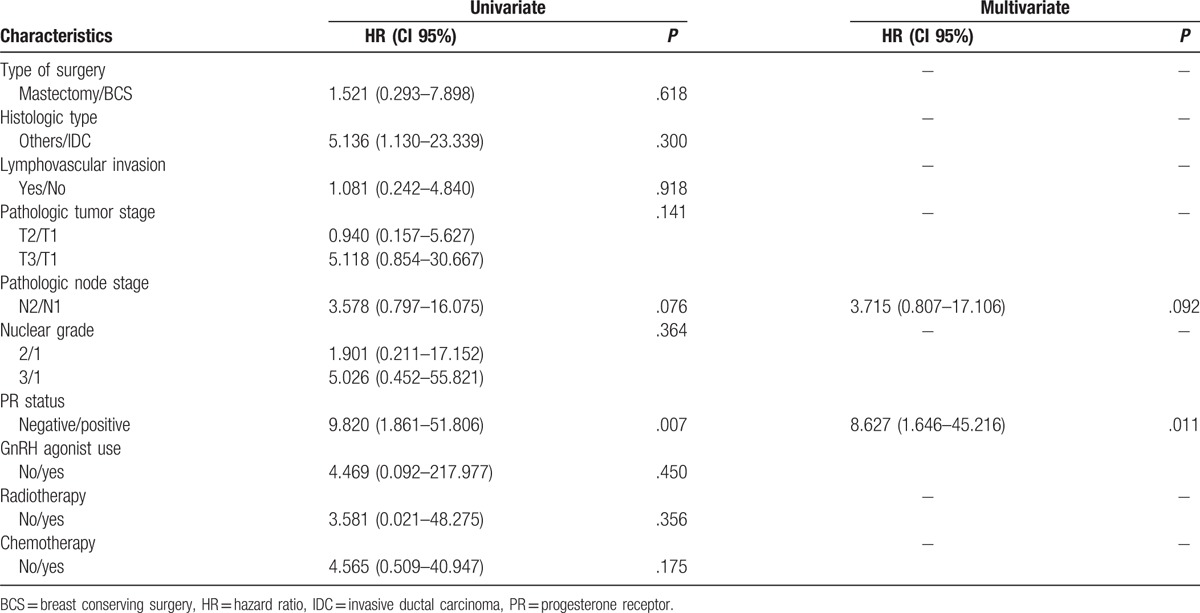
Table 3.
Univariate and multivariate Cox regression analysis for disease-free survival.
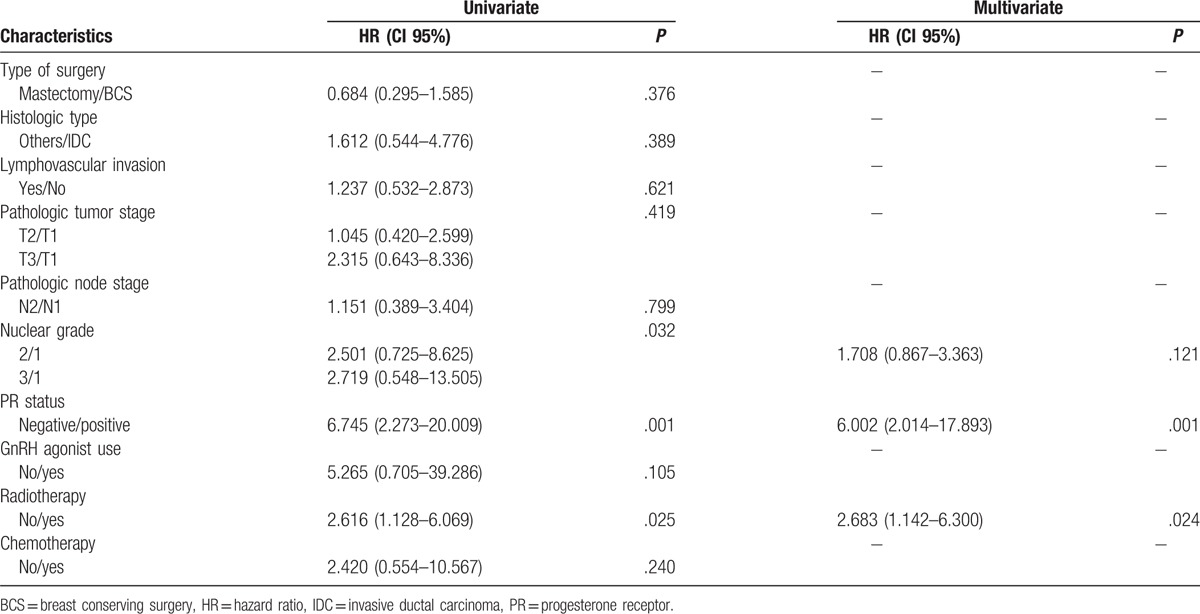
Figure 1.
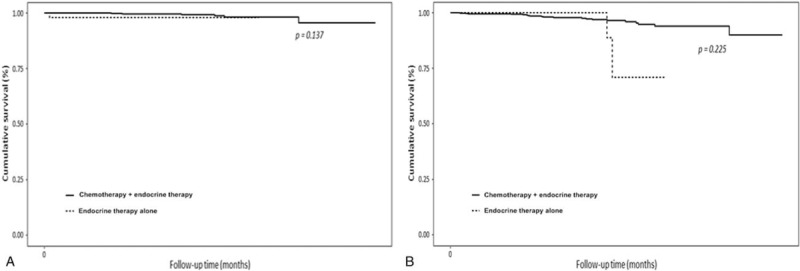
Kaplan–Meier survival curves for the overall survival rate (A), disease-free survival rate (B), between the chemotherapy group and the endocrine therapy alone group.
4. Discussion
In this study, there were no significant differences in OS and DFS between the endocrine therapy alone group and the chemotherapy group. In multivariate analysis, axillary lymph node metastasis and PR status were independent factors that affected OS between the 2 groups. Lower nuclear grade, positive PR status, and use of adjuvant radiotherapy were related to higher DFS. These results indicate that some patients with node-positive luminal A cancer may not benefit from systemic chemotherapy.
An overview of results and analyses of individual trials demonstrates a highly significant benefit from chemotherapy compared to not receiving chemotherapy.[8] In trials on anthracycline-based chemotherapy versus no chemotherapy among both women with ER-positive disease, chemotherapy reduced breast cancer mortality both in older women aged 55 to 69 years and in women less than 55 years of age.[3]
However, after the characteristics of luminal A disease were accepted by the 2011 St. Gallen panel to adequately define this subtype for clinical purposes,[1] a retrospective analysis of National Surgical Adjuvant Breast and Bowel Project (NSABP) B20 and Southwest Oncology Group (SWOG) 8814 suggested that not all patients with ER-positive disease benefit from the addition of chemotherapy. Both studies supported the use of OncotypeDX 21-gene recurrence score (RS) to define patients requiring multi-agent chemotherapy. In NSABP B20, a trial on node-negative breast cancer, patients with low-RS (<18) tumors did not benefit from chemotherapy.[10] The SWOG 8814 study demonstrated no benefit from the addition of cyclophosphamide, doxorubicin, and fluorouracil chemotherapy to tamoxifen among postmenopausal women with node-positive disease for those with ER+ and HER2- patients and specifically for those with low or intermediate RS, including luminal A disease.[11]
The primary objective of The Trial Assigning IndividuaLized Options for Treatment (TAILORx) was to determine whether adjuvant hormonal therapy was inferior to adjuvant chemotherapy plus hormonal therapy for patients with ER-positive/HER2-negative breast cancer with intermediate OncotypeDx RS values between 11 and 25.[12] The SWOG S1007 trial Treatment for Positive Node, Endocrine Responsive Breast Cancer (RxPONDER) extended the assessment to node-positive disease and determined the effects of endocrine therapy versus endocrine therapy plus chemotherapy in patients with node-positive breast cancer who do not have high RSs.[13] A nonrandomized cohort study of 106 patients with 1 to 3 positive nodes included a subset of patients who were identified as low risk by the 70-gene profile; patients who received chemotherapy had similar survival to those who did not.[14] From these multigene assays, we could presume that there would be some patients with in the ER-positive, node-positive breast cancer who would not respond to chemotherapy.[15,16] In a study of paclitaxel in node-positive cancer, paclitaxel did not benefit patients with ER-positive, HER2-negative cancers, which accounted for more than 50% of the study group.[17] In this study, AC-based chemotherapy appeared to not have a significant benefit with regard to OS (P = .137) and DFS (P = .225) in luminal A breast cancer. These results suggest that some patients with node-positive luminal A cancer will not benefit from systemic chemotherapy.
Axillary lymph node (ALN) metastasis is an accurate prognostic factor for recurrence risk and survival in patients with invasive breast cancer.[18,19] A recent study also showed that ALN metastasis was an independent prognostic factor affecting DFS.[20] In this study, ALN metastasis was significantly different in terms of OS and DFS between the 2 groups.
Our study showed that PR-positive cancer had a better prognosis with regard to OS and DFS and this result corresponded with previous studies.[21,22] Women with ER-positive/PR-negative, ER-negative/PR-positive, or ER-negative/PR-negative tumors experienced a higher risk of mortality compared with women with ER-positive/PR-positive tumors, independent of various demographic and clinical tumor characteristics.[21] Semiquantitative IHC expression of PR adds prognostic value within the current IHC-based luminal A definition by improving the identification of good outcome breast cancers.[22]
A recent study indicated that IHC subtyping was prognostic for ipsilateral breast relapse (IBR), but was not predictive of benefit from RT and patients with negative axillary nodes, so low-risk luminal A breast cancer may not need radiation therapy following breast-conserving surgery due to the low risk of ipsilateral breast relapse.[23] In contrast to the previous study, the results of our multivariable analysis in patients with positive axillary nodes indicated that DFS had a clinically significant association with radiotherapy (HR = 2.683; P = .024).
There were a few limitations of our study that need to be addressed. First, several unknown confounding factors could have affected the results owing to the retrospective study design. Second, a relatively small number of patients were enrolled in the endocrine therapy group. Last, after propensity score matching, 47 of 50 patients from the endocrine therapy alone group were N1, and 20 patients had micrometastasis of lymph nodes. Therefore, the majority of the endocrine therapy alone group was at low risk of recurrence and that might have influenced the results. Nevertheless, the results of this study have clinical significance and provide important insight regarding the use of adjuvant systemic chemotherapy in node-positive, luminal A breast cancer.
In conclusion, adjuvant chemotherapy could provide little benefit to postmenopausal patients with luminal A, node-positive breast cancer and endocrine therapy alone may help reduce morbidity. Future studies with a large number of patients and longer follow-up time are necessary to determine whether chemotherapy might be avoided in this patient population.
Footnotes
Abbreviations: AC = doxorubicin plus cyclophosphamide, ALN = axillary lymph node, ALND = axillary lymph node dissection, CAF = cyclophosphamide, doxorubicin, 5-fluorouracil, CI = confidence intervals, DFS = disease-free survival, EBC = early breast cancer, ER = estrogen receptor, FISH = fluorescence in situ hybridization, GnRH = gonadotropin-releasing hormone, HER2 = human epidermal growth factor receptor 2, HR+ = hormone receptor-positive, IBR = ipsilateral breast relapse, IHC = immunohistochemistry, NSABP = National Surgical Adjuvant Breast and Bowel Project, OS = overall survival, PR = progesterone receptor, RS = recurrence score, RxPONDER = Treatment for Positive Node, Endocrine Responsive Breast Cancer, SISH = silver in situ hybridization, SWOG = Southwest Oncology Group, T = taxanes, TAILORx = Trial Assigning IndividuaLized Options for Treatment.
SP and SKL shared co-first authorship.
Funding: This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C3418) and by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (2016R1A5A2945889).
The authors have no conflicts of interest to disclose.
References
- [1].Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gnant M, Steger GG. Fighting overtreatment in adjuvant breast cancer therapy. Lancet 2009;374:2029–30. [DOI] [PubMed] [Google Scholar]
- [3].Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Carrick S, Parker S, Wilcken N, et al. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev 2009;2:CD003372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].O'Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 2002;20:2812–23. [DOI] [PubMed] [Google Scholar]
- [6].Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol 2003;21:588–92. [DOI] [PubMed] [Google Scholar]
- [7].NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast cancer Version 1. 2016. The National Comprehensive Cancer Network. Available at: www.nccn.org. 2016. Accessed December 21, 2015. [Google Scholar]
- [8].Group EBCTC. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687–717. [DOI] [PubMed] [Google Scholar]
- [9].Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726–34. [DOI] [PubMed] [Google Scholar]
- [11].Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol 2008;26:721–8. [DOI] [PubMed] [Google Scholar]
- [13].Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials 2013;34:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mook S, Schmidt MK, Viale G, et al. The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1-3 positive lymph nodes in an independent validation study. Breast Cancer Res Treat 2009;116:295–302. [DOI] [PubMed] [Google Scholar]
- [15].Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med 2009;360:790–800. [DOI] [PubMed] [Google Scholar]
- [16].Albain KS, Paik S, van’t Veer L. Prediction of adjuvant chemotherapy benefit in endocrine responsive, early breast cancer using multigene assays. Breast 2009;18(suppl 3):S141–5. [DOI] [PubMed] [Google Scholar]
- [17].Hayes DF. Targeting adjuvant chemotherapy: a good idea that needs to be proven!. J Clin Oncol 2012;30:1264–7. [DOI] [PubMed] [Google Scholar]
- [18].Recht A, Houlihan MJ. Axillary lymph nodes and breast cancer: a review. Cancer 1995;76:1491–512. [DOI] [PubMed] [Google Scholar]
- [19].Jatoi I, Hilsenbeck SG, Clark GM, et al. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol 1999;17:2334–40. [DOI] [PubMed] [Google Scholar]
- [20].Kwak HY, Chae BJ, Eom YH, et al. Is adjuvant chemotherapy omissible in women with T1-2 stage, node-positive, luminal A type breast cancer? J Chemother 2015;27:290–6. [DOI] [PubMed] [Google Scholar]
- [21].Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 2007;9:R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Prat A, Cheang MC, Martin M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 2013;31:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu FF, Shi W, Done SJ, et al. Identification of a low-risk luminal a breast cancer cohort that may not benefit from breast radiotherapy. J Clin Oncol 2015;33:2035–40. [DOI] [PubMed] [Google Scholar]


