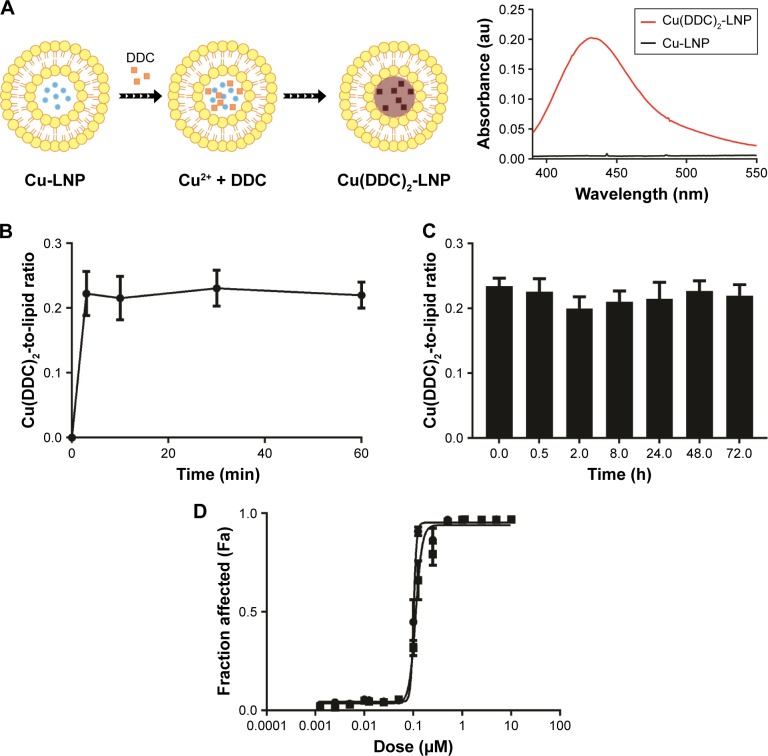
Figure 1.
Characterization of Cu(DDC)2 prepared in a liposomal formulation (DSPC/Chol (55:45)) wherein the Cu(DDC)2 was synthesized inside the copper-containing liposomes.
Notes: (A) Schematic representation of Cu(DDC)2 complex formation inside copper liposomes upon addition of DDC. Cu-liposomes and Cu(DDC)2-liposomes were scanned on a UV-Vis spectrophotometer, and the formation of Cu(DDC)2 results in a peak at 435 nm indicative of complex formation. (B) Formation of Cu(DDC)2 inside DSPC/Chol liposomes (final liposomal lipid concentration was 20 mM) as a function of time over 1 h at 25°C following addition of DDC to the liposomes at a final DDC-to-lipid ratio of 0.4. (C) Cu(DDC)2 release from DSPC/Chol (55:45) liposomes over a 72-h time course in the presence of 50% FBS at 37°C. Cu(DDC)2 was measured using a UV-Vis spectrophotometric assay and lipid was measured through use of a radioactive lipid marker (3H-CHE). (D) MV-4–11 cytotoxicity curves for Cu(DDC)2 dissolved in DMSO (▪) and the Cu(DDC)2 liposomal preparation (•) where cell viability was measured (using PrestoBlue) following a 72-h exposure to the added Cu(DDC)2. Data are presented as mean ± standard error of the mean of 3 experiments. If error bars are not seen then they are within the size of the symbol used.
Abbreviations: Chol, cholesterol; DDC, diethyldithiocarbamate; DSPC, distearoyl-sn-glycero-3-phosphocholine; FBS, fetal bovine serum; 3H-CHE, 3H-cholesteryl hexadecyl ether; UV-Vis, ultraviolet-visible; DMSO, dimethyl sulfoxide.