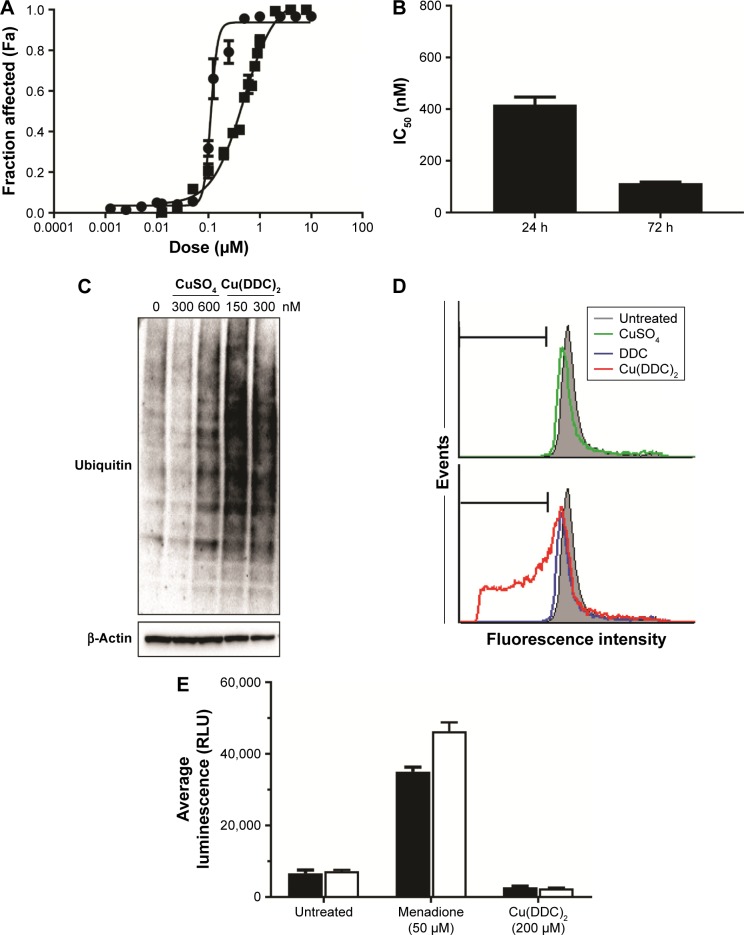
Figure 2.
Cu(DDC)2 acts primarily as a proteosome inhibitor and induces cell death in MV-4–11 cells.
Notes: (A) Cytotoxicity curves generated when MV-4–11 cells are exposed to Cu(DDC)2 (prepared inside DSPC/Chol liposomes) for either 24 (▪) or 72 (•) h where viability was measured using PrestoBlue. (B) The IC50 values of MV-4–11 cells that were treated with Cu(DDC)2 for 24 and 72 h. The proteasome inhibition activity was determined as described in the Methods using MV-4–11 cells treated with the indicated doses of CuSO4 or Cu(DDC)2 (prepared inside DSPC/Chol liposomes) for 24 h. (C) Proteasome inhibition resulted in accumulation of ubiquitinated proteins presented as long dark bands on the Western blot following Cu(DDC)2 treatment (150 and 300 nM) but not for vehicle or CuSO4 (300–600 nM). (D) Cell-cycle analyses were completed using MV-4–11 cells treated with CuSO4, DDC, or Cu(DDC)2 for 24 h and the results indicated no significant change in the cell cycle upon Cu(DDC)2 exposure. There was an increase in the sub G0/G1 fraction (marked with horizontal bar) when cells were treated with Cu(DDC)2, indicative of cell death as evident by DNA fragmentation. (E) ROS formation was tested in MV-4–11 cells treated with Cu(DDC)2 (prepared inside DSPC/Chol liposomes), where ROS formation was measured 4 h following initiation of treatment. Cu(DDC)2 treatment did not induce ROS formation. Menadione was used as a positive control and ROS formation in the cells was evident by a statistically significant difference in luminescence relative to the corresponding cell-free condition. Data are presented as mean ± standard error of the mean of 3 experiments.
Abbreviations: Chol, cholesterol; DDC, diethyldithiocarbamate; DSPC, distearoyl-sn-glycero-3-phosphocholine; ROS, reactive oxygen species.