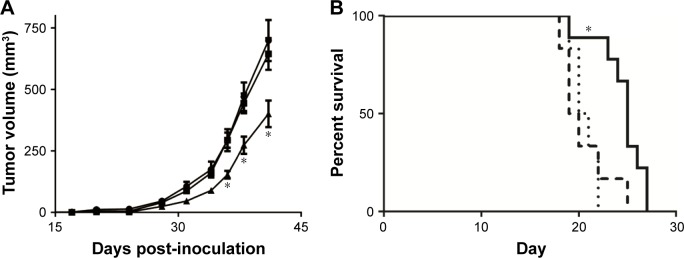
Figure 4.
Cu(DDC)2 efficacy studies.
Notes: (A) MV-4–11 cells (1×106) were inoculated subcutaneously into RAG-2M mice and the tumors were treated intravenously with vehicle (SH buffer, •), copper-containing liposomes (1.3 mg/kg, ▪) or Cu(DDC)2 (8 mg/kg, ▴) formulated in DSPC/Chol liposomes using a M, W, F ×2 dosing schedule. Tumor volumes were measured 3 times a week and are plotted as mean ± standard error of the man (up to 9 mice per group). As shown, there was a significant delay in tumor growth when comparing tumor sizes in animals that were treated with Cu(DDC)2 to those treated with the vehicle control. “*” indicates statistically significant difference (P<0.05) upon statistical analysis using a two-way analysis of variance followed by Tukey’s adjustments to correct for multiple comparisons. (B) The activity of Cu(DDC)2 (prepared in DSPC/Chol liposomes) was evaluated following CED of Cu(DDC)2 in a rat glioma model wherein 10,000 F98 cells were implanted intracranially into the right caudate nucleus. A single 10 µL injection of vehicle (SH buffer, ·····), copper liposomes (0.08 mg/mL, – –), or Cu(DDC)2 (0.5 mg/mL, —) was injected 10 days after F98 implantation (n=9). Survival study statistical analysis was performed using the log rank test with GraphPad Prism 6. *A P-value under 0.05 was considered statistically significant.
Abbreviations: Chol, cholesterol; CED, Convection-enhanced delivery; DDC, diethyldithiocarbamate; DSPC, distearoyl-sn-glycero-3-phosphocholine; M, W, F ×2, Monday, Wednesday, and Friday for 2 weeks; SH, sucrose HEPES.