Abstract
Emotion dysregulation (ED) reflects deficits in understanding and managing negative emotions and may serve as a transdiagnostic mechanism of risk for trauma-related psychiatric disorders. Therefore, understanding neurobiological substrates of ED in traumatized individuals is critical. The present study examined associations between ED and baseline structural differences and patterns of functional activity during an emotional task in a sample of African American women (n = 136) recruited from an urban hospital. Participants engaged in a structural magnetic resonance imaging (MRI) session. A subsample (n = 92) also viewed emotional face stimuli during functional MRI. ED was related to greater dorsal anterior cingulate cortex (dACC) surface area (Pcorr < 0.05) and increased dorsomedial prefrontal cortex (dmPFC) and ventromedial PFC activation to fearful stimuli (Pcorr < 0.05), independent of the trauma and psychiatric symptoms. DMPFC activation was also associated with posttraumatic stress disorder and depression symptoms. Mediation analyses showed a significant mediation effect of ED on the relation between dmPFC activation and psychiatric symptoms. These findings are important since dACC and dmPFC play central roles in fear expression and attention to emotional stimuli. Future longitudinal research is needed to help solidify a model of risk for how such neural substrates may be impacted by traumatic experiences to create ED.
Keywords: emotion dysregulation, trauma, magnetic resonance imaging, post-traumatic stress, disorder, depression
Introduction
Difficulty with emotion regulation is a central component of many psychiatric disorders (Gross and Munoz, 1995; Aldao et al., 2010). Emotion dysregulation (ED) includes problems with emotional awareness or acceptance, difficulty controlling behavior in the presence of strong emotions, and an inability to adaptively use strategies to manage intense negative emotions. Exposure to trauma, particularly early in life, has been shown to be a major risk factor for the development of ED (Cicchetti et al., 1995; Maughan and Cicchetti, 2002; Alink et al., 2009). In turn, ED is seen as a risk factor for many trauma-related psychiatric disorders, including post-traumatic stress disorder (PTSD) and depression, suggesting that it is an important mechanism of risk linking early life trauma and later psychiatric outcomes (Shields and Cicchetti, 2001; Kim and Cicchetti, 2010). Understanding the neurobiological substrates of ED in the context of trauma exposure is critical in growing efforts to move away from a focus on distinct diagnoses and identify underlying mechanisms of risk, which may be specifically targeted in the context of treatment.
Great strides have been made in understanding the brain regions involved in the broader prefrontal-limbic network implicated in emotional processing and regulation. Limbic regions including amygdala and insula have been associated with emotional reactivity, while prefrontal regions are involved in emotional awareness, experience and regulation (Etkin et al., 2015). Among prefrontal regions, dorsomedial prefrontal cortex (dmPFC) and dorsal anterior cingulate cortex (dACC) help with emotional awareness, track level of emotional arousal (Lane et al., 1998) and are activated by emotional conflict (Etkin et al., 2006). Dorsolateral and ventrolateral PFC assist in directly modifying emotional experience (explicit regulation) and rostral ACC and ventromedial PFC produce reflexive emotion regulation processes that may occur in the absence of deliberate attempts to control emotions (implicit regulation; Etkin and Wager, 2007).
Efforts to understand brain structural markers and functional activation patterns associated with the broader prefrontal-limbic network related to emotion regulation in healthy individuals has focused primarily on specific emotion regulation strategies (i.e. cognitive reappraisal and emotional suppression; see Ochsner and Gross, 2005; Cutuli, 2014, for reviews) as well as implicit emotion regulation (Etkin et al., 2010; Gyurak et al., 2011). Greater reappraisal use is associated with greater volume in prefrontal regions (dACC, Giuliani et al., 2011; vmPFC, Welborn et al., 2009), and with the amygdala (Hermann et al., 2013). Expressive suppression use is also positively associated with volume in dACC and dmPFC (Hermann et al., 2013; Kühn et al., 2011), but negatively associated with vmPFC (Welborn et al., 2009). Regarding neural correlates, increased activation in prefrontal regions (dACC, vmPFC and dlPFC) and reduced activity in limbic regions (amygdala), in response to emotional stimuli have been implicated in successful cognitive reappraisal (Goldin et al., 2008; Vanderhasselt et al., 2013). For expressive suppression, activation in both PFC regions (vlPFC, dlPFC medial PFC) and limbic regions (insula and amygdala) have been found (Goldin et al., 2008; Vanderhasselt et al., 2013). To summarize, the ACC, PFC, insula and amygdala appear to play an important role in emotion regulation, yet variations of gray matter volume and patterns of brain function that contribute to dysregulated emotion more generally, thus not specific to a single emotion regulation strategy, have yet to be fully understood.
Research on structural or functional differences in this prefrontal-limbic network in relation to psychiatric disorders can also inform our understanding of brain correlates of ED. Indeed, differences in the structure of ACC and amygdala have also been associated with major depressive disorder (MDD; Bora et al., 2012; van Eijndhoven et al., 2013) and PTSD (Yamasue et al., 2003; Woodward et al., 2006; Rogers et al., 2009; Thomaes et al., 2010). There is some evidence of increased thickness of dACC and decreased thickness of rACC in MDD patients compared with controls (Bora et al., 2012; van Eijndhoven et al., 2013). Both decreased ACC and amygdala volume have been associated with PTSD (Yamasue et al., 2003; Woodward et al., 2006; Rogers et al., 2009; Thomaes et al., 2010). Altered activation patterns in PFC, ACC and amygdala have also been implicated in both depression (Siegle et al., 2002, 2007; Anand et al., 2005; Yoshimura et al., 2010) and PTSD (Felmingham et al., 2008; Lanius et al., 2010, 2012). In depressed patients, studies show hyperactivity in the mPFC (Yoshimura et al., 2010), dlPFC (Anand et al., 2005), ACC (Anand et al., 2005; Yoshimura et al., 2010) and amygdala (Siegle et al., 2002, 2007) during emotional tasks. Research on PTSD suggests different types of functional activity patterns based on the type of ED present. Under-modulation of negative emotion (i.e. presence of re-experiencing and hyperarousal PTSD symptoms) is associated with low activation of the vmPFC and rACC and high activation in the amygdala during emotion-eliciting tasks. Over-modulation of negative emotion, which is often thought of as a marker for dissociation, is associated with abnormally high activation of the medial PFC and dACC (Felmingham et al., 2008; Lanius et al., 2010, 2012). It is important to note that trauma exposure was not always accounted for in the context of these studies, particularly in the depressed samples.
Given the well-established association between trauma exposure and ED, it would be valuable to study these neural substrates in the context of a traumatized population. Therefore, the goal of this study was to identify associations between ED and both baseline structural differences and patterns of functional activity during an emotional face viewing task in a sample of women with high rates of exposure to trauma (including early life trauma) and high levels of psychiatric symptoms. Given the above described prior research, brain structure analyses were conducted using the amygdala, dACC, rACC, dmPFC and vmPFC as a priori regions of interest (ROI). We predicted that ED would be associated with lower amygdala volume, lower thickness and/or surface area of the rACC and vmPFC, and greater thickness and/or surface area of the dACC and dmPFC. Patterns of functional activity were also assessed using a whole brain analysis approach to capture the range of functional regions associated with various emotion regulation strategies. Secondary analyses were also conducted to determine the unique association between ED and these regions above and beyond the effects of trauma exposure and current psychiatric symptoms (i.e. PTSD, depression). Although not a direct aim of the study, analyses will be conducted to replicate previous findings of the association between trauma exposure, ED and psychiatric symptoms (PTSD, depression) within this sample.
Methods
Participants
Participants were recruited through an ongoing study of risk factors for PTSD. Participants were approached in the general medical clinics of Grady Memorial Hospital, a publicly funded hospital that serves economically disadvantaged individuals in Atlanta, Georgia. Consistent with the population of patients being seen in the hospital, the majority of participants in this study are African-American (>90%). For this research we only included female subjects with self-reported African-American race/ethnicity to enhance data homogeneity, in addition to the fact that this is an under-represented group in psychiatric imaging research studies. Men were not included in this study, as significant sex differences have been observed in the neural processing of emotional stimuli (Mak et al., 2009; Stevens and Hamann, 2012). All participants were screened and met the inclusion criteria: between ages 18 and 65 and able to provide informed consent; exclusion criteria included presence of current psychosis, neurological disorders1, current psychotropic medication or any MRI contraindications (e.g. ferromagnetic implants). Participants had normal or corrected-to-normal vision. Urine tests for pregnancy and non-prescribed substance use were conducted 24 h prior to MRI scan, and individuals who showed positive results for pregnancy or non-prescribed substances were excluded. All participants provided written informed consent prior to participating. Participants received monetary compensation for their time. The institutional review board of Emory University approved the study procedures, and testing took place at Grady Memorial Hospital and the Biomedical Imaging Technology Center at Emory University Hospital.
The sample included 136 African-American women (mean age = 38.83, s.d. = 11.84) who completed the structural MRI scan and all self-report measures used in the study. In the overall sample, 32.4% were employed and 83.5% reported a household income of <$2000 a month. Almost all participants were exposed to trauma (95.6%). A subset of those women (n = 92) also completed the functional fMRI task. There were no significant differences in demographics or variables of interest across the two groups.
Measures
Psychological measures were administered on the day of recruitment.
Emotion d ysregulation. The Emotion Dysregulation Scale (EDS), is a 12-item self-report scale of ED (Powers et al., 2015) and was used to measure current level of ED. Items are scored from ‘1 (not true)’ to ‘7 (very true)’ and assess domains of emotional experiencing (e.g. ‘Emotions overwhelm me’), cognition (e.g. ‘When I’m upset, everything feels like a disaster or crisis’), and behavior (e.g. ‘When my emotions are strong, I often make bad decisions’). In this sample, the average score on the EDS was 35.90 (s.d. = 18.94, range = 12–84). Internal consistency of the EDS was high (α = 0.93) and the measure has shown good construct validity within this population in predicting a range of negative psychiatric outcomes (Powers et al., 2015).
Psychiatric s ymptoms. The PTSD Symptom Scale (Foa and Tolin, 2000) is an 18-item self-report scale and was used to assess current PTSD symptoms (17 items) and duration (mean = 12.48, s.d. = 11.81, range = 0–50). Diagnosis of PTSD was based on DSM-IV-TR (APA, 2000) diagnostic criteria for PTSD, and requires report of at least one re-experiencing symptom, two avoidance/numbing symptoms and two hyperarousal symptoms, as well as symptom duration of at least 1 month (31.3% of women in the sample met criteria for PTSD). The Beck Depression Inventory, II (BDI; Beck et al., 1996) is a 21-item self-report measure and was used to assess current depression symptoms (mean = 15.06, s.d. = 11.46, range = 0–48). Diagnosis of depression was based on a score of 18 or higher (32.8% of women in this sample met criteria for depression).
Trauma e xposure. The Childhood Trauma Questionnaire (CTQ; Bernstein et al., 2003) is a 25-item self-report instrument assessing sexual, physical, emotional abuse, and neglect in childhood (mean = 40.75, s.d. = 16.84, range = 25–105). Based on cutoffs established by Bernstein and Fink (1998) for moderate-to-severe reported exposure to emotional (score ≥ 13), physical (score ≥ 10) and/or sexual (score ≥ 8) abuse in childhood, 48.2% of our participants experienced moderate-to-severe abuse.
The Traumatic Events Inventory (TEI; Gillespie et al., 2009) is a 14-item screening instrument and was used to measure total level of trauma exposure by a sum score reflecting the total number of different types of trauma to which a participant had been exposed over the course of their life (child abuse was excluded from analyses to avoid overlap with CTQ; mean = 4.03, s.d. = 2.40, range = 0–11).
Fearful faces fMRI task
Eight fearful and eight neutral (four male and four female) faces were selected from the stimulus set of Ekman and Friesen (Ekman and Friesen, 1976). Blocks of fearful and neutral stimuli (15 blocks each) were presented in a pseudorandom order. Participants were instructed to pay attention to the faces, but did not make any behavioral response, in order to minimize motion artifacts and neural activation unrelated to processing the visual stimulus (for full procedure, see Stevens et al., 2013).
Brain imaging acquisition and analysis
Brain imaging data were acquired on a Siemens 3.0-Tesla Magnetom Trio TIM whole-body MR scanner (Siemens, Malvern, PA) using a 12-channel head coil. Structural images were acquired using a gradient-echo, T1-weighted pulse sequence (TR = 2600 ms, TE = 3.02 ms; 1 × 1 × 1 mm voxel size). Functional images were acquired using the Z-SAGA pulse sequence (Heberlein and Hu, 2004) to minimize signal loss due to susceptibility artifacts. Each scan volume contained 30 axially acquired 4 mm thick images with an in-plane resolution of 3.44 × 3.44 mm utilizing the parameters: TR = 3000 ms, TE1 = 30 ms, TE2 = 67 ms, at a flip angle of 90°.
Structural MRI analysis. Both cortical thickness and surface area were examined. T1 images were processed in Freesurfer version 5.3 (https://surfer.nmr.mgh.harvard.edu). Gray matter volume from subcortical structures was extracted through automated segmentation, and data quality checks were performed following the ENIGMA 2 protocol (http://enigma.ini.usc.edu/protocols/imaging-protocols/), a method designed to standardize quality control procedures across laboratories to facilitate replication. Amygdala volume and total intracranial volume were exported to SPSS for further analysis.
Cortical surface reconstruction and thickness measurement were performed using Freesurfer’s surface-based analysis, and data quality checks were performed following the ENIGMA 3 protocol. A priori regions were exported to SPSS for further analysis. These regions included: left and right dACC (Freesurfer label: caudal ACC), left and right rACC (Freesurfer label: rostral ACC), left and right dmPFC (Freesurfer label: superior frontal) and left and right vmPFC (Freesurfer label: medial orbitofrontal). See Supplementary Materials for additional details.
FMRI data analysis. Preprocessing and statistical analysis was conducted in SPM8 (Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm). ArtRepair software (Mazaika et al., 2007) was used to correct signal spike and motion artifacts. Slices containing spike artifacts were identified and replaced using linear interpolation, with no >5% of slices repaired per participant. Volumes affected by motion artifact were repaired using linear interpolation, with no >5% of volumes repaired per participant. Slice timing correction and spatial realignment were applied to the functional images. A 128 Hz high-pass filter was used to remove low-frequency noise (Holmes et al., 1997). The anatomical image was then co-registered to the mean functional image and normalized to the International Consortium for Brain Mapping 152-subject template. The resulting normalization parameters were applied to the functional images. The functional images were then smoothed with an 8 mm Gaussian kernel.
Hemodynamic responses to blocks of fearful and neutral stimuli were modeled with a boxcar function representing the onset and 8000 ms duration of the block, convolved with a canonical hemodynamic response function, as implemented in SPM8. Participant-specific motion parameters were included as covariates. Contrast images representing the linear comparison of beta values for the fearful vs neutral conditions were constructed for each participant, and were entered into group-level random effects analysis to identify clusters of significant activation. Group-level models were covaried for age.
The effect of EDS total score on brain activation was assessed voxel-wise across the whole brain. Statistical analyses were conducted using permutation testing implemented in the Statistical nonParametric Mapping toolbox (SnPM13; http://warwick.ac.uk/snpm). Permutation approaches have recently been shown to provide an ideal balance of Types I and II error in simulation studies (Eklund et al., 2015). Results were corrected to P < 0.05, FWE-corrected, with an initial threshold of P < 0.001 and 5000 permutations. For clusters meeting corrected brain-wide significance, contrast estimates for the fearful > neutral contrast were extracted as the mean of all super-threshold voxels, for use in secondary analyses implemented in SPSS.
Data analytic approach
First, correlations between EDS score, trauma variables and psychiatric symptoms were assessed, as well as analysis of variance for mean differences in EDS score based on PTSD and depression diagnoses. Then, correlations between brain structural ROIs and EDS score were conducted. Amygdala, dACC, rACC, dmPFC and vmPFC (left and right hemispheres assessed separately) were used as ROIs based on previous research findings associating these regions with emotion regulation and trauma-related psychopathology relevant to ED. Bonferroni correction was used to account for multiple testing across the ROIs (18 correlation analyses total).
For the subset of women (n = 92) with available fMRI data, the mean contrast values for the clusters that met whole brain significance were extracted. A series of multiple linear regression models were utilized to examine the unique predictive value of these patterns of activation (i.e. contrast values) on ED symptoms, over and above trauma exposure, PTSD symptoms and depression symptoms. Secondary analyses were then conducted to examine associations between contrast values for patterns of activation and psychiatric outcomes (i.e. current PTSD and depression severity). Then, based on these results, the potential mediating effect of EDS score on associations between significant clusters and psychiatric outcomes was assessed. Mediation analyses were performed with PROCESS (Hayes, 2013) for SPSS. We generated 5000 bootstrap samples to yield a 95% bias-corrected confidence interval (CI) of the indirect effect a×b (i.e. the mediation effect). If the CI for the indirect effect (a×b) calculated by the bootstrap analysis does not include zero, the indirect effect is significant and mediation is established.
Results
Associations between ED, trauma exposure and clinical outcomes
There were strong positive correlations between EDS score and child abuse severity (r136= 0.35, P < 0.001), overall trauma exposure (r136 = 0.21, P < 0.01), current PTSD symptoms (r136= 0.48, P < 0.001), and depression symptoms (r136= 0.56, P < 0.001). When examined by depression or PTSD diagnoses, ANOVA results demonstrated significantly higher levels of ED symptoms in participants with depression [F= 48.18, P < 0.001; mean (s.d.): no depression: 29.30 (15.90), depression: 49.83 (17.81)] and PTSD [F= 26.48, P < 0.0001; mean (s.d.): no PTSD: 30.92 (15.63), PTSD: 47.29 (21.27)].
Structural analysis of brain regions with EDS
As shown in Table 1, right dACC surface area average was significantly positively associated with EDS after controlling for multiple testing using Bonferroni correction (r136= 0.26, P = 0.002). No other regions examined were significantly correlated with EDS. Based on the correlation results, a secondary linear regression analysis was used to determine whether surface area of the right dACC was predictive of EDS score above and beyond the effects of age, ICV, child abuse severity, lifetime trauma load (excluding child abuse), PTSD symptoms and depression symptoms. The overall model including all variables was significant (F change = 9.39, P < 0.01) and accounted for 42% of the variance in EDS score, with right dACC surface area accounting for 4% of unique variance above and beyond trauma and psychopathology (b= 0.22, t= 3.07, P < 0.01).
Table 1.
Correlations between EDS score and volume, surface area, and thickness Freesurfer data
| EDS score | |
|---|---|
| r-value | |
| Brain volume: | |
| Amygdala (L) | −0.03 |
| Amygdala (R) | −0.04 |
| Surface area: | |
| dACC (L) | 0.15 |
| dACC (R) | 0.26** |
| rACC (L) | 0.05 |
| rACC (R) | 0.03 |
| dmPFC (L) | −0.01 |
| dmPFC (R) | −0.03 |
| vmPFC (L) | 0.11 |
| vmPFC (R) | 0.02 |
| Thickness: | |
| dACC (L) | −0.13 |
| dACC (R) | 0.06 |
| rACC (L) | −0.04 |
| rACC (R) | −0.14 |
| dmPFC (L) | −0.07 |
| dmPFC (R) | −0.07 |
| vmPFC (L) | 0.02 |
| vmPFC (R) | 0.01 |
P = 0.002 (the Bonferroni correction for significance).
fMRI activation with EDS in response to fearful facial expressions
Whole-brain analyses showed significant positive correlations between EDS and reactivity to fearful face stimuli in the superior temporal gyrus/interior frontal gyrus (BA38/47; cluster 1), the dmPFC (BA9; cluster 2), vmPFC (BA11; cluster 3) and middle temporal gyrus (BA37/21; cluster 4) regions when controlling for multiple testing (see Figure 1 and Table 2).
Fig. 1.
Activation results of region response to fearful stimuli with ED scores based on whole brain analysis, Pcorr < 0.05. Results are displayed in neurological orientation on a representative single-subject template brain in MNI space. Scatter plot graph shows the correlation between mean contrast estimate across voxels in the dmPFC cluster, for the Fear > Neutral contrast, and ED score.
Table 2.
Whole-brain analysis characterizing significant associations between region activation and EDS score
| MNI coordinates |
||||||||
|---|---|---|---|---|---|---|---|---|
| Cluster | Region | Side | X | Y | Z | mm3 | T value | FWEcorr |
| P-value | ||||||||
| 1 | Superior Temporal Gyrus, Inferior Frontal Gyrus (BA 38, 47) | L | −42 | 20 | −22 | 3264 | 4.35 | 0.032 |
| 2 | dmPFC: Superior Medial Frontal Gyrus, Medial Frontal Gyrus (BA 9) | L | −2 | 48 | 26 | 2304 | 4.32 | 0.049 |
| 3 | vmPFC: Orbital Medial Frontal Gyrus, Medial Frontal Gyrus (BA 11) | L | −2 | 52 | −14 | 2496 | 4.25 | 0.045 |
| 4 | Middle Temporal Gyrus (BA 37, 21) | L | −62 | −24 | −14 | 4928 | 4.09 | 0.018 |
n = 92; Brodmann area (BA).
Linear regression analyses were then conducted to evaluate the predictive value of fMRI contrast values for the four significant clusters on EDS score above and beyond the effects of age, childhood abuse severity, lifetime trauma load, current PTSD and depression symptom severity. As shown in Table 3, clusters were all significantly predictive of EDS beyond the effects of covariates (P < 0.01).
Table 3.
Linear regression model predicting EDS score from significant clusters
| R2 | t | R2 change | F change | |
|---|---|---|---|---|
| Model 1 | 0.47 | 0.47 | 14.15*** | |
| Model 2 | ||||
| Superior Temporal Gyrus/Inferior Frontal Gyrus (Cluster 1) | 0.58 | 5.17 | 0.13 | 26.68*** |
| dmPFC (Cluster 2) | 0.51 | 3.28 | 0.06 | 10.75** |
| vmPFC (Cluster 3) | 0.54 | 4.08 | 0.9 | 16.62*** |
| Middle Temporal Gyrus (Cluster 4) | 0.56 | 4.63 | 0.11 | 21.41*** |
P < 0.01; ***P < .001.
Model 1 includes covariates age, child abuse severity score (CTQ), non-abuse trauma exposure (TEI), current PTSD symptoms (modified PTSD Symptoms Scale), and depression symptoms (BDI); Model 2 includes covariates in step 1 and contrast values for the four significant clusters entered into separate models.
Associations between activated brain regions and psychiatric outcomes
Secondary analyses were then conducted to determine whether these clusters were associated with psychiatric outcomes (i.e. current PTSD and depression symptoms). Of the four clusters, only dmPFC (Cluster 2) was significantly associated with PTSD (r92= 0.26, P = 0.014) and depression symptoms (r92= 0.26, P = 0.014). See Supplementary Table S1 for bivariate correlations between clusters 1–4 and psychiatric outcomes.
Based on the significant relationships between the dmPFC and these psychiatric outcomes, two mediation analyses were run to determine whether ED mediated the association between dmPFC activation and both psychiatric outcomes. As shown in Figure 2, EDS score fully mediated the association between dmPFC reactivity to faces and current PTSD symptoms (overall model: R2= 0.20, F= 11.21, P < 0.001, bias corrected 95% CI = 3.64–14.56) as well as current depression symptoms (overall model: R2= 0.40, F= 30.10, P < 0.001, 95% CI = 6.62–19.42).
Fig. 2.
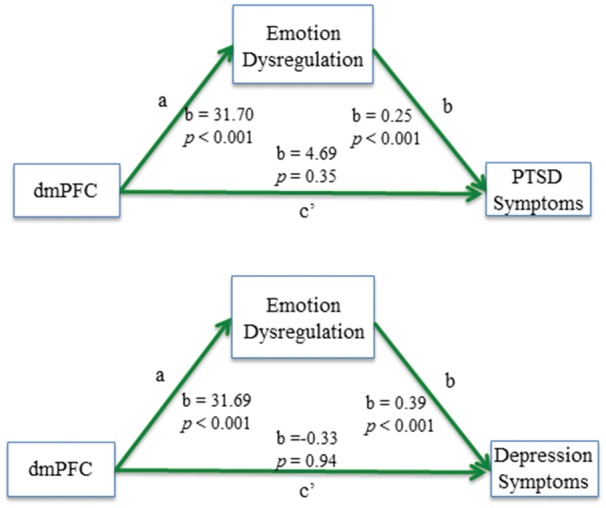
PROCESS mediation model showing the full mediating effect of ED on the relation between dmPFC activation during a fearful faces task and current PTSD and depression symptoms; a, association between dmPFC activation and ED score; b, association between ED score and PTSD/depression symptoms; c’, association between dmPFC and PTSD/depression symptoms once ED is included in the mediation model.
Discussion
In this study, we investigated associations between ED symptoms and brain structure and functional activity in an urban, community sample of African American women with high rates of trauma exposure and psychiatric symptoms. Across both structural and functional analysis, we found that ED was independently related to patterns of structural difference and functional activity in brain regions associated with emotion processing and learning above and beyond any effects of trauma exposure or current PTSD or depression symptoms. Although not a specific aim of this study, in support of the growing body of research showing that ED is a transdiagnostic factor related to many types of psychiatric disorders (Southam-Gerow and Kendall, 2002; Aldao et al., 2010; Bradley et al., 2011), we also found strong relationships between ED, trauma exposure and PTSD and depression symptoms in this sample further demonstrating these important associations.
Regarding structural analytic results, in support of our hypothesis, we found evidence of a positive association between ED symptoms and right dACC surface area, an area well known for its role in processing emotional content and fear learning (e.g. Milad et al., 2009; Etkin et al., 2011). Other ROI examined (amygdala, rACC, dmPFC, vmPFC) were not significantly associated with self-reported ED. Research examining structural markers of ED among participants with significant levels of psychiatric symptoms is very limited, and studies examining structural abnormalities in MDD and PTSD, whose symptoms involve features of ED, have been mixed. Some evidence in MDD patients has shown greater thickness of the dACC in patients compared with healthy controls (van Eijndhoven et al., 2013). However, there has been more consistent evidence that lower volume of rACC is associated with exposure to child abuse, MDD and anxiety disorders (Koolschijn et al., 2009; van Tol et al., 2010; Bora et al., 2012; Korgaonkar et al., 2013), as well as lower dACC volume in patients with child-abuse related complex PTSD (Thomaes et al., 2010). Interestingly, in healthy adults, greater dACC thickness is positively related to skin conductance response during fear conditioning; given its role in fear expression, the structure of dACC may predispose individuals to have stronger fear reactions (Milad et al., 2007). There is growing evidence that supports the idea of use-dependent brain changes, such that volumetric changes can occur based on usage (Maguire et al., 2000; Gaser and Schlaug, 2003; Draganski et al., 2004). Therefore, it is possible that greater surface area in the dACC is a reflection of greater use of this region and greater negative emotion expression. Alternatively, greater surface area in this region may be a predisposing factor that impacts fear expression and over-attention to emotional cues in the environment for individuals, making them more susceptible to develop significant deficits in emotion regulation. In fact, structural differences in brain regions do not necessarily explain functional differences in brain activity, and so it will be critical to examine the development and impact of such structural changes on ED in future longitudinal research.
Regarding functional activity, we found significant associations between ED symptoms and activation to fearful faces in the superior temporal and inferior frontal gyri (BA38/47), dmPFC (BA9), vmPFC (BA11) and middle temporal gyrus (BA37/21). Importantly, linear regression analyses demonstrated that these clusters significantly predicted ED symptoms even after accounting for trauma exposure and psychiatric symptoms (effect sizes ranged from 0.06 to 0.13). The finding that individuals with high levels of ED show increased dmPFC response to fearful stimuli is consistent with research showing dmPFC activation when individuals are asked to purposefully increase their emotional response to a stimulus (Ochsner et al., 2004). BA 9 itself is associated with processing of emotional scenes and attention to negative emotions (Lane et al., 1997; Bermpohl et al., 2006). This suggests that greater activation of the dmPFC during this task may represent a pattern of overactive attention to processing negative emotional stimuli (even when the threat is low) in individuals with high levels of ED, leaving fewer cognitive resources available to engage in other processes, such as adaptive emotion regulation. Additionally, activation of vmPFC has previously been associated with implicit emotion regulation (Etkin et al., 2006, 2011) and the use of reappraisal and suppression (Goldin et al., 2008; Vanderhasselt et al., 2013). Therefore, these women with high levels of ED may also be attempting to regulate emotional reactions but are expending significant resources without actual effectiveness in adaptively modulating the emotional experience.
We also found that dmPFC was associated with current PTSD and depression symptom severity within this sample. Our finding further supports previous research showing hyper-activation of dmPFC and dACC in individuals with PTSD (Lanius et al., 2010; Rougemont‐Bücking et al., 2011). Findings suggest that the function of this region may be impaired in the processing of contextual information in individuals with PTSD (Milad et al., 2009; Rougemont-Bucking et al., 2011). For example, in a study of fear learning and extinction in PTSD patients (Rougemont‐Bücking et al., 2011), the investigators found that dmPFC activation tracked the likelihood of threat within a particular context, and individuals with PTSD did modulate their dmPFC responses to a context that signaled safety. There is other evidence that dmPFC and dACC activation during tasks involving attention to emotional cues could be representative of attentional bias for threat in individuals with PTSD (Fani et al., 2012). However, since we also found that the dmPFC region was associated with current depressive symptoms, the findings suggest that this region may be important to risk for or resilience to multiple negative psychiatric outcomes. In fact, we found that ED symptoms fully mediated the association between activity in the dmPFC and these psychiatric outcomes, suggesting that the link between heightened dmPFC activation during processing of negative emotional stimuli and psychiatric outcomes in this sample is a result of ED, not disorder-specific psychiatric symptoms per se.
Clearly, none of these brain regions discussed operate in isolation and are all part of a broader network that impact emotional experiences and responses (see Etkin and Wager, 2007 for a broader review of this network in PTSD). Among healthy individuals, PFC and ACC help to monitor and resolve emotional conflict and response, dampening limbic activation through top-down inhibition. Evidence shows hyperactivation of limbic regions and hypoactivation of PFC and ACC regions in individuals with PTSD, and communication between those regions within the network also breaks down (Stevens et al., 2013). Examination of connectivity between regions was beyond the scope of the present study, but future studies should examine connectivity between PFC, ACC and limbic regions in traumatized adults with high and low levels of ED and psychiatric symptoms.
Despite evidence suggesting that the PFC modulates limbic regions (including the amygdala) and that this circuit plays a central role in emotion regulation (Stein et al., 2007), we did not find any evidence for an association between ED and amygdala activation. Previous neuroimaging studies have shown that dmPFC activation correlates positively with cognitive emotion regulation (i.e. appraisal) and amygdala activation correlates negatively with cognitive emotion regulation (Ochsner et al., 2002; Etkin et al., 2011), but we could not replicate this finding, potentially due to the conservative correction for multiple testing that we applied. Research has consistently shown that PTSD is associated with increased amygdala response to fearful stimuli (e.g. Shin et al., 2005; Bryant et al., 2008; Fonzo et al., 2010) and this has been shown within our lab using a similar sample (Stevens et al., 2013). Although this heightened amygdala response may represent heightened emotional reactivity in individuals with PTSD, anxiety disorders and depression (Etkin and Wager, 2007; Siegle et al., 2007), our measure of ED reflects more generalized problems, such as managing strong emotions as they arise and feeling out of control with one’s own emotion.
Thus, overactivation of dmPFC may better capture a transdiagnostic risk factor for deficits in the regulation of emotional response that also spans multiple disorders. All the data used in this study are cross sectional, such that clear pathways of risk cannot be established at this time. This question would be best addressed by a longitudinal investigation examining whether dmPFC (and amygdala) reactivity before development of ED or exposure to traumatic events is predictive of later development of ED and subsequent PTSD and depression post-trauma.
This study included several limitations. First, all psychiatric variables of interest were measured using self-report. Future studies would benefit from creating symptom severity scores and diagnosis for PTSD and depression through clinical interviews. Using a longer measure of ED that includes various dimensions of ED would be beneficial. Including behavioral and physiological measures of ED in addition to self-report may also help to further disentangle the brain mechanisms involved in ED. In addition, the participants viewed stimuli that depicted Caucasian faces, raising the possibility that social out-group biases may influence neural responses. The primary reason for selecting this task was that it has been well validated in the literature (e.g. Breiter et al., 1996; Shin et al., 2005). However, future work should examine neural responses to faces representing African American and other racial/ethnic groups. Additionally, we did not assess for how current life stress related to financial and environmental factors may independently impact the associations found. Finally, this study included only female African American participants; the findings may not generalize to men or other racial groups. Although generalizability to populations other than African American women in is a concern, this is off set by the fact than African American women participants are underrepresented in this area of research. Further, data on this understudied population have public health importance, given the high levels of trauma and trauma-associated health problems present in this population Furthermore, most of the research on emotion regulation is completed in samples comprised of healthy individuals as well as those with limited trauma exposure, and little remains known about ED in either functional or structural MRI research in the context of traumatized populations. Therefore, there is in fact a great benefit in conducting research in this high-risk population.
In summary, the current findings demonstrate that ED is associated with increased dACC surface area and enhanced vmPFC and dmPFC activation among civilian African American women drawn from a highly traumatized population. Furthermore, ED mediates associations between enhanced dmPFC activation and depression and PTSD, showing evidence of its role as a transdiagnostic factor associated with many forms of psychopathology and the potential independent neurobiological risk factors associated with this construct. These results provide support for a model of risk in which neural substrates supporting healthy emotional processing are disrupted and may negatively impact emotion regulation abilities, thus leading to various psychiatric outcomes. Future longitudinal research is needed to further understand this pathway of risk.
Funding
This work was primarily supported by the National Institute of Mental Health (MH071537; MH102890, MH101976) and the National Institute of Child Health and Human Development (HD071982). Support also included Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01 RR00039), the Emory Medical Care Foundation and the Burroughs Welcome Fund.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
Footnotes
Screening for previous closed head injury with loss of consciousness was based on self-report.
References
- Aldao A., Nolen-Hoeksema S., Schweizer S. (2010). Emotion-regulation strategies across psychopathology: a meta-analytic review. Clinical Psychology Review, 30, 217–37. [DOI] [PubMed] [Google Scholar]
- Alink L., Cicchetti D., Kim J., Rogosh F. (2009). Mediating and moderating processes in the relation between maltreatment and psychopathology: Mother-child relationship quality and emotion regulation. Journal of Abnormal Child Psychology, 37(6), 831–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y., et al. (2005). Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological Psychiatry, 57(10), 1079–88. [DOI] [PubMed] [Google Scholar]
- Beck A., Steer R., Brown G. (1996) Manual for the Beck Depression Inventory-II, San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bermpohl F., Pascual‐Leone A., Amedi A., et al. (2006). Attentional modulation of emotional stimulus processing: an fMRI study using emotional expectancy. Human Brain Mapping, 27(8), 662–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D., Fink L. (1998) Manual for the childhood trauma questionnaire, New York: The Psychological Corporation. [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., et al. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect, 27(2), 169–90. [DOI] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yücel M. (2012). Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. Journal of Affective Disorder, 138(1), 9–18. [DOI] [PubMed] [Google Scholar]
- Bradley B., DeFife J., Guarnaccia C., Phifer J., Fani N., Ressler K. (2011). Emotion dysregulation and negative affect: association with psychiatric symptoms. Journal of Clinical Psychiatry, 72, 685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter H.C., Etcoff N.L., Whalen P.J., et al. (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17(5), 875–87. [DOI] [PubMed] [Google Scholar]
- Bryant R.A., Kemp A.H., Felmingham K.L., et al. (2008). Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Human Brain Mapping, 29(5), 517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D., Ackerman B., Izard C. (1995). Emotions and emotion regulation in developmental psychopathology. Development and Psychopathology, 7(1), 1–10. [Google Scholar]
- Cutuli D. (2014). Cognitive reappraisal and expressive suppression strategies role in the emotion regulation: an overview on their modulatory effects and neural correlates. Frontiers in Systems Neuroscience 8, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Busch V., Schuierer G., Bogdahn U., May A. (2004). Neuroplasticity: changes in grey matter induced by training. Nature, 427(6972), 311–2. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T., Knutsson H. (2015). Can parametric statistical methods be trusted for fMRI based group studies?. arXiv Preprint arXiv, 1511.01863. [Google Scholar]
- Ekman P., Friesen W. V. (1976) Measuring facial movement. Environmental psychology and nonverbal behavior, 1(1), 56–75.
- Etkin A., Büchel C., Gross J.J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16(11), 693–700. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D., Kandel E., Hirsch J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51, 871–82. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Hoeft F., Menon V., Schatzberg A.F. (2010). Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry 167(5), 545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., Jovanovic T., Ely T.D., et al. (2012). Neural correlates of attention bias to threat in post-traumatic stress disorder. Biological Psychology, 90(2), 134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K., Kemp A., Williams L., et al. (2008). Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychological Medicine, 38(12), 1771–80. [DOI] [PubMed] [Google Scholar]
- Foa E.B., Tolin D.F. (2000). Comparison of the PTSD symptom scale–interview version and the clinician-administered PTSD scale. Journal of Traumatic Stress, 13(2), 181–91. [DOI] [PubMed] [Google Scholar]
- Fonzo G.A., Simmons A.N., Thorp S.R., Norman S.B., Paulus M.P., Stein M.B. (2010). Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry, 68(5), 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C., Schlaug G. (2003). Brain structures differ between musicians and non-musicians. The Journal of Neuroscience, 23(27), 9240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie C., Bradley B., Mercer K., et al. (2009). Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry, 31, 505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N.R., Drabant E.M., Gross J.J. (2011). Anterior cingulate cortex volume and emotion regulation: is bigger better? Biological Psychology, 86(3), 379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6), 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Munoz R. (1995). Emotion regulation and mental health. Clinical Psychology: Science and Practice, 2, 151–64. [Google Scholar]
- Gyurak A., Gross J. J., Etkin A. (2011). Explicit and implicit emotion regulation: a dual-process framework. Cognition and Emotion, 25(3), 400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. (2013) Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, New York: Guilford Press. [Google Scholar]
- Heberlein K.A., Hu X. (2004). Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magnetic Resonance in Medicine, 51(1), 212–6. [DOI] [PubMed] [Google Scholar]
- Hermann A., Bieber A., Keck T., Vaitl D., Stark R. (2013). Brain structural basis of cognitive reappraisal and expressive suppression. Social Cognitive and Affective Neuroscience, 9(9), 1435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A., Josephs O., Buchel C., Friston K. (1997). Statistical modelling of low-frequency confounds in fMRI. NeuroImage, 5, S480. [Google Scholar]
- Kim J., Cicchetti D. (2010). Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychol Psychiatry, 51(6), 706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P., van Haren N.E., Lensvelt‐Mulders G.J., Pol H., Hilleke E., Kahn R.S. (2009). Brain volume abnormalities in major depressive disorder: a meta‐analysis of magnetic resonance imaging studies. Human Brain Mapping, 30(11), 3719–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar M.S., Antees C., Williams L.M., et al. (2013). Early exposure to traumatic stressors impairs emotional brain circuitry. PLoS One, 8(9), e75524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Gallinat J., Brass M. (2011). ‘Keep calm and carry on’: structural correlates of expressive suppression of emotions. PLoS One, 6(1), e16569.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R.D., Reiman E.M., Axelrod B., Yun L.S., Holmes A., Schwartz G.E. (1998). Neural correlates of levels of emotional awareness: evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 10, 525–35. [DOI] [PubMed] [Google Scholar]
- Lane R.D., Reiman E.M., Bradley M.M., et al. (1997). Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia, 35(11), 1437–44. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Brand B., Vermetten E., Frewen P.A., Spiegel D. (2012). The dissociative subtype of posttraumatic stress disorder: Rationale, clinical and neurobiological evidence, and implications. Depress Anxiety, 29(8), 701–8. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Vermetten E., Loewenstein R.J., et al. (2010). Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. The American Journal of Psychiatry, 167(6), 640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E.A., Gadian D.G., Johnsrude I.S., et al. (2000). Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America, 97(8), 4398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak A.K., Hu Z., Zhang J.X., Xiao Z., Lee T.M. (2009). Sex-related differences in neural activity during emotion regulation. Neuropsychologia, 47(13), 2900–8. [DOI] [PubMed] [Google Scholar]
- Maughan A., Cicchetti D. (2002). Impact of child maltreatment and interadult violence on children’s emotion regulation abilities and socioemotional adjustment. Child Development, 73(5), 1525–42. [DOI] [PubMed] [Google Scholar]
- Mazaika P., Whitfield-Gabrieli S., Reiss A. (2007). Artifact repair for fMRI data from high motion clinical subjects. Human Brain Mapping. [Google Scholar]
- Milad M.R., Pitman R.K., Ellis C.B., et al. (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry, 66(12), 1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J., Pitman R.K., Orr S.P., Fischl B., Rauch S.L. (2007). A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry, 62(10), 1191–4. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. (2002). Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–29. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–9. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Knierim K., Ludlow D.H., et al. (2004). Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience, 16(10), 1746–72. [DOI] [PubMed] [Google Scholar]
- Powers A., Stevens J., Fani N., Bradley B. (2015). Construct validity of a short, self report instrument assessing emotional dysregulation. Psychiatry Research, 225(1), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M.A., Yamasue H., Abe O., et al. (2009). Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Research: Neuroimaging, 174(3), 210–6. [DOI] [PubMed] [Google Scholar]
- Rougemont‐Bücking A., Linnman C., Zeffiro T.A., et al. (2011). Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neuroscience and Therapeutics, 17(4), 227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Cicchetti D. (2001). Parental maltreatment and emotion dysregulation as risk factors for bullying and victimization in middle childhood. Journal of Clinical Child Psychology, 30(3), 349–63. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Wright C.I., Cannistraro P.A., et al. (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry, 62(3), 273–81. [DOI] [PubMed] [Google Scholar]
- Siegle G.J., Steinhauer S.R., Thase M.E., Stenger V.A., Carter C.S. (2002). Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry, 51(9), 693–707. [DOI] [PubMed] [Google Scholar]
- Siegle G.J., Thompson W., Carter C.S., Steinhauer S.R., Thase M.E. (2007). Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry, 61(2), 198–209. [DOI] [PubMed] [Google Scholar]
- Southam-Gerow M., Kendall P. (2002). Emotion regulation and understanding: implications for child psychopathology and therapy. Clinical Psychology Review, 22, 189–222. [DOI] [PubMed] [Google Scholar]
- Stein J.L., Wiedholz L.M., Bassett D.S., et al. (2007). A validated network of effective amygdala connectivity. Neuroimage, 36(3), 736–45. [DOI] [PubMed] [Google Scholar]
- Stevens J.S., Hamann S. (2012). Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia, 50(7), 1578–93. [DOI] [PubMed] [Google Scholar]
- Stevens J.S., Jovanovic T., Fani N., et al. (2013). Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research, 47(10), 1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K., Dorrepaal E., Draijer N., et al. (2010). Reduced anterior cingulate and orbitofrontal volumes in child abuse–related complex PTSD. Journal of Clinical Psychiatry, 71(12), 1478–644. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P., van Wingen G., Katzenbauer M., et al. (2013). Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. American Journal of Psychiatry, 170(12), 1477–86. [DOI] [PubMed] [Google Scholar]
- van Tol M.J., van der Wee N.J., van den Heuvel O.A., et al. (2010). Regional brain volume in depression and anxiety disorders. Archives of General Psychiatry, 67(10), 1002–11. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt M.A., Baeken C., Van Schuerbeek P., Luypaert R., De Raedt R. (2013). Inter-individual differences in the habitual use of cognitive reappraisal and expressive suppression are associated with variations in prefrontal cognitive control for emotional information: an event related fMRI study. Biological Psychology, 92(3), 433–9. [DOI] [PubMed] [Google Scholar]
- Welborn B.L., Papademetris X., Reis D.L., Rajeevan N., Bloise S.M., Gray J.R. (2009). Variation in orbitofrontal cortex volume: relation to sex, emotion regulation and affect. Social Cognitive and Affective Neuroscience, 4, 328–39, nsp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward S.H., Kaloupek D.G., Streeter C.C., Martinez C., Schaer M., Eliez S. (2006). Decreased anterior cingulate volume in combat-related PTSD. Biological Psychiatry, 59(7), 582–7. [DOI] [PubMed] [Google Scholar]
- Yamasue H., Kasai K., Iwanami A., et al. (2003). Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proceedings of the National Academy of Sciences of the United States of America, 100(15), 9039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S., Okamoto Y., Onoda K., Matsunaga M., Ueda K., Suzuki S. (2010). Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. Journal of Affective Disorders, 122(1), 76–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



