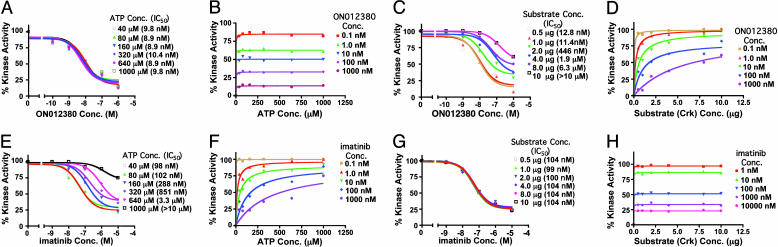
Fig. 2.
Steady-state kinetic analysis of BCR-ABL kinase inhibition by ON012380. (A) BCR-ABL kinase inhibition assays were performed as described for Fig. 1 in a reaction mixture containing [γ-32P]ATP and various concentrations (conc.) of ATP. The values from individual samples were analyzed and plotted as a function of inhibitor concentration. The IC50 of ON012380 for kinase activity was calculated. (B) The curves represent calculated best fits to the Michaelis–Menton equation with a constant amount of substrate and various amounts of ATP and ON012380. (C) BCR-ABL kinase inhibition assays with different concentrations of ON012380 and various concentrations of substrate (Crk) were performed, and the values from individual samples were analyzed and plotted as a function of inhibitor concentration. (D) Michaelis–Menton curves for BCR-ABL with a curve fit derived by using nonlinear regression analysis is shown for data obtained by using a constant amount of ATP and various amounts of substrate and ON012380. (E) Inhibition assays with recombinant BCR-ABL protein and different concentrations of imatinib were performed in the presence of various concentrations of ATP as described for A. (F) The curves represent calculated best fits to the Michaelis–Menton equation with a constant amount of substrate and various amounts of ATP and imatinib. (G) Inhibition assays with recombinant BCR-ABL protein and different concentrations of imatinib were performed in the presence of various concentrations of substrate (Crk) as described for E, and the values from individual samples were analyzed and plotted as a function of drug concentration. (H) The curves represent calculated best fits to the Michaelis–Menton equation with a constant amount of ATP and various amounts of substrate and imatinib.