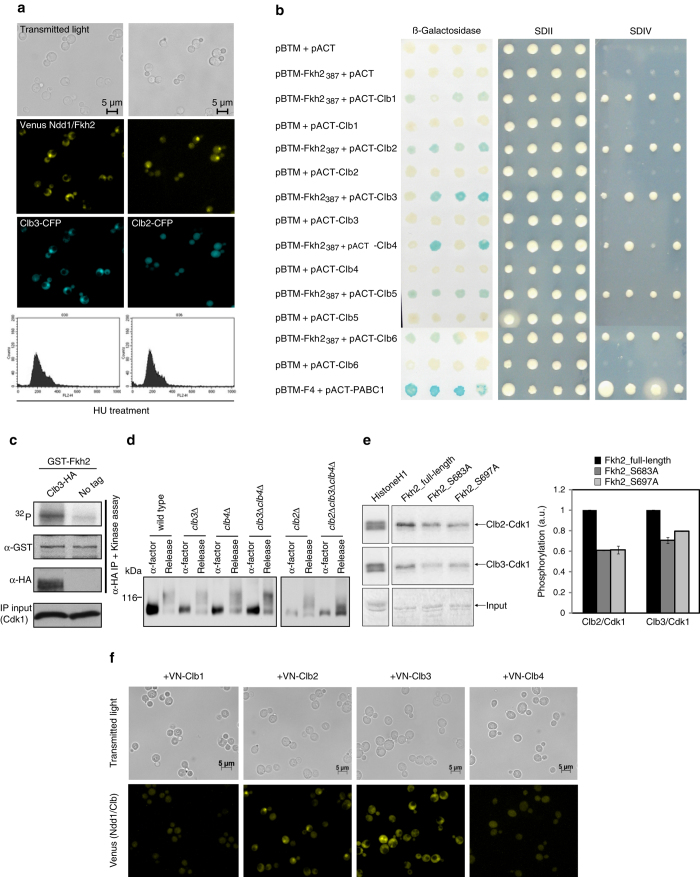
Fig. 3.
Clb3/Cdk1 co-localizes with, interacts and phosphorylates the Ndd1/Fkh2 complex. a Co-colocalization of Clb3 and Clb2 with the Ndd1/Fkh2 complex. Yeast cells expressing Ndd1-VC and carrying Clb3-CFP or Clb2-CFP integrated in the genome were transformed with the plasmid p426-VN-Fkh2. Selected transformants were synchronized in S phase with hydroxyurea (HU) and monitored for the fluorescent (BiFC) signal. A co-localization with the BiFC signal is shown for Clb3. Growth arrest was examined by staining with propidium iodide and FACS analysis. b Fkh2 C-terminal region (Fkh2387) associates with Clb cyclins by Yeast-two-Hybrid assay. Yeast cells expressing the fusion proteins LexA-Fkh2387 (pBTM-Fkh2387) and AD-Clb1-6 (pACT-Clb1-6) were spotted onto SDII and SDIV selective media or on a membrane for detection of the β-galactosidase activity. The interaction between ATXN2-FD and PABC was used as positive control.12 At least three independent transformations have been performed, and four clones were tested in each experiment. c Fkh2 is a substrate of Clb3/Cdk1 kinase activity in vitro. Yeast lysate expressing Clb3-HA was incubated with GST-Fkh2 expressed and purified from E. coli. After the kinase assay, the membrane was plotted using antibodies against GST (to detect Fkh2), HA (to detect Clb3), and PSTAIR (to detect Cdk1). The accuracy of the result was verified by monitoring that a similar amount of Cdk1 kinase in the immunoprecipitation (IP), and of GST-Fkh2 was used in the assay. d Fkh2 is phosphorylated in vivo by Clb3/Cdk1 kinase activity. Wild type, clb3Δ, clb4Δ, clb3Δclb4Δ, clb2Δ, and clb2Δclb3Δclb4Δ were grown at 25 °C in YPD medium and then synchronized in G1 phase with α-factor, before releasing them into fresh medium for 50 min (Release). Protein extracts were analyzed in 6% polyacrylamide gel electrophoresis (PAGE) 10 μM Phos-tag gel and Fkh2-6HA was followed by western blot. e Fkh2 phosphosite specificity of Clb3/Cdk1. Fkh2 full-length and mutants (S684A and S697A) were isolated from bacterial protein extracts and phosphorylated by Clb2/Cdk1 and Clb3/Cdk1 isolated from yeast extracts containing overexpressed Clb2-TAP or Clb3-TAP. An excess of purified Fkh2 was added to fully saturate the cyclin/Cdk1 complexes. Kinase reactions were started by adding purified Clb2/Cdk1 or Clb3/Cdk1, and 32P-ATP to the mixture. Histone H1 was used as a control, and standard sodium dodecyl sulfate-PAGE was used to separate phosphorylated species. Quantification of the phosphorylation extent (arbitrary units) is realized with PhosphoImager, as average from two different time points; values relative to a full-length Fkh2 are shown. The result is representative of three independent experiments. f Clb3 associates with Ndd1 by BiFC. Yeast cells expressing the fusion protein Ndd1-VC were transformed with the plasmids p426-VN-Clb1, p426-VN-Clb2, p426-VN-Clb3, and p426-VN-Clb4, respectively. Detection of the fluorescent (BiFC) signal was revealed by fluorescence microscopy