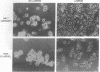
Abstract
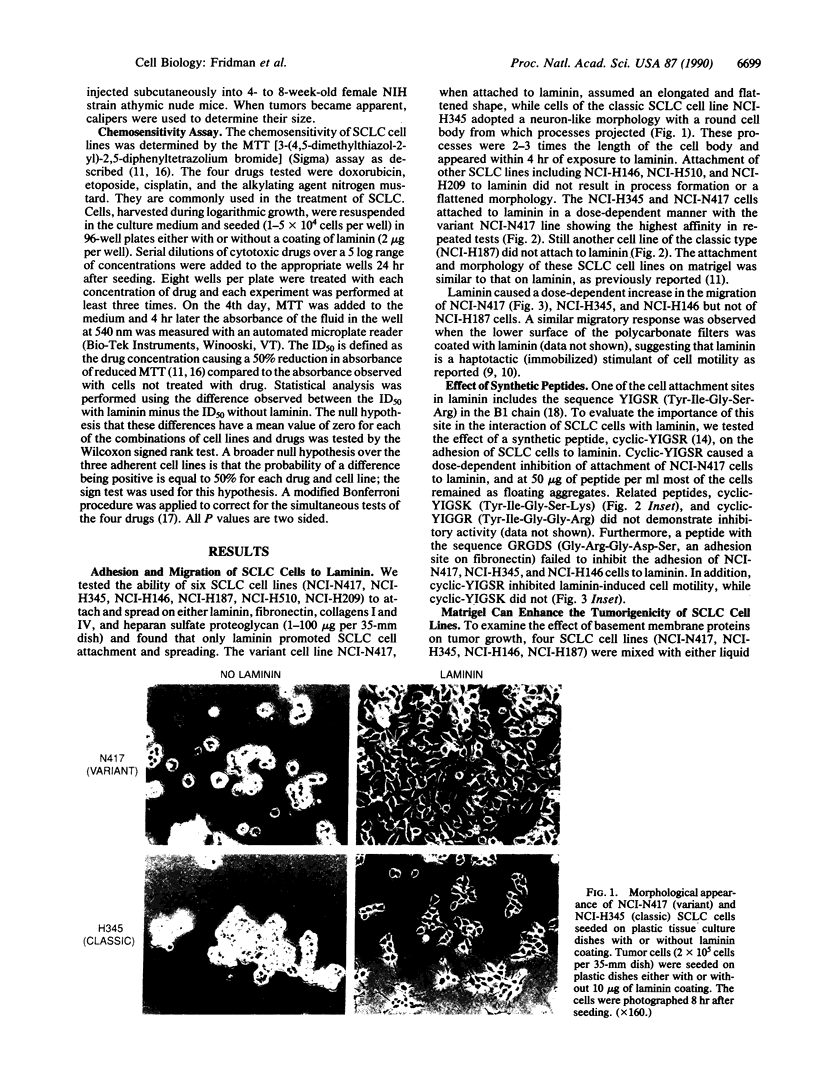
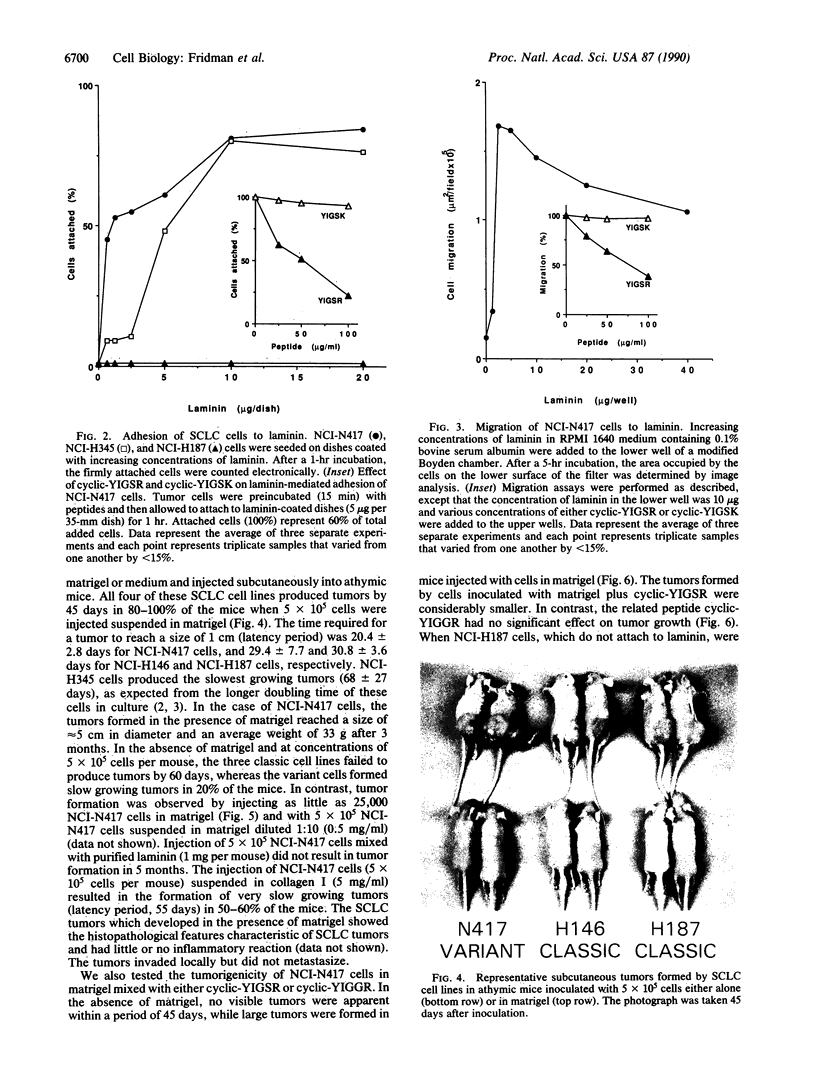
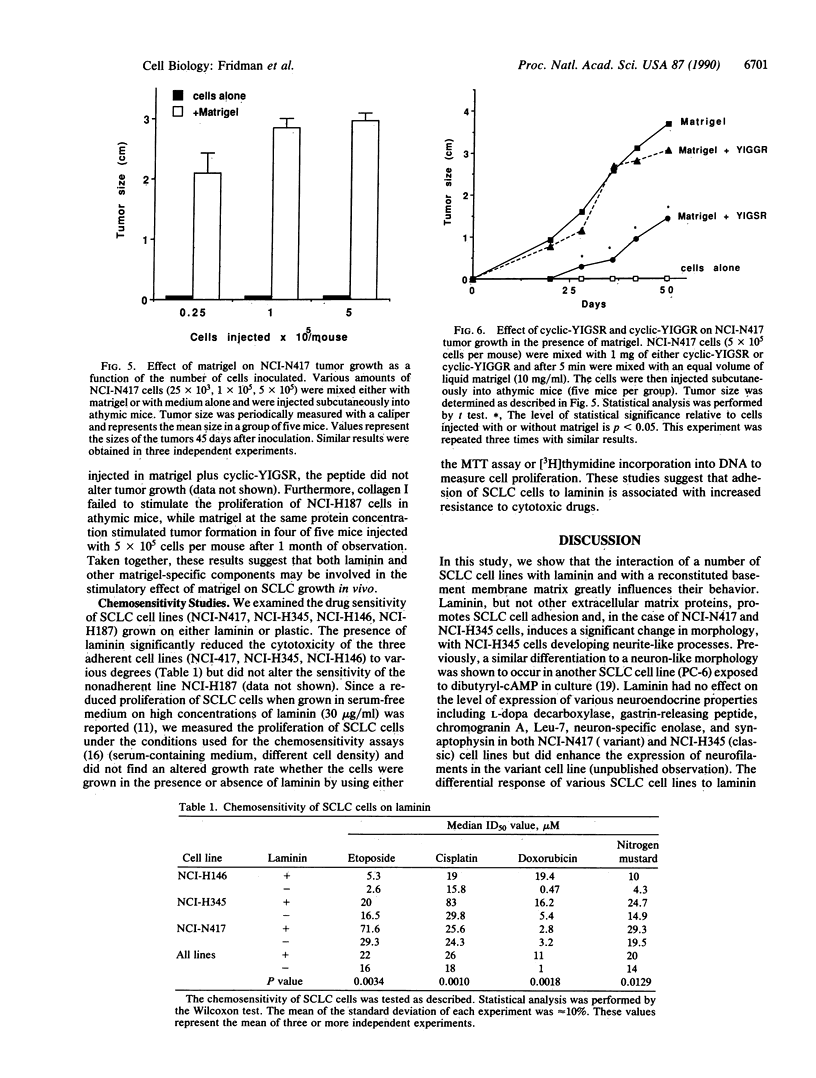
Small cell lung cancer (SCLC) is a fatal malignancy due to its propensity to metastasize widely and to reoccur after chemotherapy in a drug-resistant form. While most SCLC cell lines are anchorage independent for growth, laminin induced the attachment of five of six SCLC cell lines tested (NCI-N417, NCI-H345, NCI-H146, NCI-H187, NCI-H510, and NCI-H209). NCI-N417 SCLC cells adopted a flattened morphology on laminin, and a classic SCLC cell line (NCI-H345) demonstrated a neuron-like appearance while the other SCLC cell lines except NCI-H187 cells, attached but did not spread. Adhesion to laminin was associated with increased resistance to several cytotoxic drugs. Matrigel, an extract of basement membrane proteins, greatly accelerated tumor growth when coinjected with SCLC cells in athymic mice. A synthetic peptide from the B1 chain of laminin, cyclic-YIGSR (Tyr-Ile-Gly-Ser-Arg), inhibited laminin-induced SCLC cell adhesion and migration in vitro and reduced the size of the tumors they formed when coinjected with matrigel and YIGSR. These results suggest that the interaction of SCLC cells with laminin and possibly with other basement membrane proteins can enhance their tumorigenicity and drug resistance.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Carney D. N., Bunn P. A., Jr, Gazdar A. F., Pagan J. A., Minna J. D. Selective growth in serum-free hormone-supplemented medium of tumor cells obtained by biopsy from patients with small cell carcinoma of the lung. Proc Natl Acad Sci U S A. 1981 May;78(5):3185–3189. doi: 10.1073/pnas.78.5.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Nau M. M., Minna J. D. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985 Jun;45(6):2924–2930. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Sims H. L., Simmons A. Heterotransplantation of small-cell carcinoma of the lung into nude mice: comparison of intracranial and subcutaneous routes. Int J Cancer. 1981 Dec;28(6):777–783. doi: 10.1002/ijc.2910280617. [DOI] [PubMed] [Google Scholar]
- Graf J., Iwamoto Y., Sasaki M., Martin G. R., Kleinman H. K., Robey F. A., Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell. 1987 Mar 27;48(6):989–996. doi: 10.1016/0092-8674(87)90707-0. [DOI] [PubMed] [Google Scholar]
- Gunji Y., Gorelik E. Role of fibrin coagulation in protection of murine tumor cells from destruction by cytotoxic cells. Cancer Res. 1988 Sep 15;48(18):5216–5221. [PubMed] [Google Scholar]
- Kleinman H. K., Graf J., Iwamoto Y., Sasaki M., Schasteen C. S., Yamada Y., Martin G. R., Robey F. A. Identification of a second active site in laminin for promotion of cell adhesion and migration and inhibition of in vivo melanoma lung colonization. Arch Biochem Biophys. 1989 Jul;272(1):39–45. doi: 10.1016/0003-9861(89)90192-6. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Lai S. L., Goldstein L. J., Gottesman M. M., Pastan I., Tsai C. M., Johnson B. E., Mulshine J. L., Ihde D. C., Kayser K., Gazdar A. F. MDR1 gene expression in lung cancer. J Natl Cancer Inst. 1989 Aug 2;81(15):1144–1150. doi: 10.1093/jnci/81.15.1144. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Anchorage dependency effects on difluoromethylornithine cytotoxicity in human lung carcinoma cells. Cancer Res. 1986 Apr;46(4 Pt 1):1844–1848. [PubMed] [Google Scholar]
- McCarthy J. B., Basara M. L., Palm S. L., Sas D. F., Furcht L. T. The role of cell adhesion proteins--laminin and fibronectin--in the movement of malignant and metastatic cells. Cancer Metastasis Rev. 1985;4(2):125–152. doi: 10.1007/BF00050692. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Furcht L. T. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J Cell Biol. 1984 Apr;98(4):1474–1480. doi: 10.1083/jcb.98.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. B., Palm S. L., Furcht L. T. Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. J Cell Biol. 1983 Sep;97(3):772–777. doi: 10.1083/jcb.97.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLemore T. L., Liu M. C., Blacker P. C., Gregg M., Alley M. C., Abbott B. J., Shoemaker R. H., Bohlman M. E., Litterst C. C., Hubbard W. C. Novel intrapulmonary model for orthotopic propagation of human lung cancers in athymic nude mice. Cancer Res. 1987 Oct 1;47(19):5132–5140. [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Terranova V. P., Williams J. E., Liotta L. A., Martin G. R. Modulation of the metastatic activity of melanoma cells by laminin and fibronectin. Science. 1984 Nov 23;226(4677):982–985. doi: 10.1126/science.6505678. [DOI] [PubMed] [Google Scholar]
- Turpeenniemi-Hujanen T., Thorgeirsson U. P., Rao C. N., Liotta L. A. Laminin increases the release of type IV collagenase from malignant cells. J Biol Chem. 1986 Feb 5;261(4):1883–1889. [PubMed] [Google Scholar]