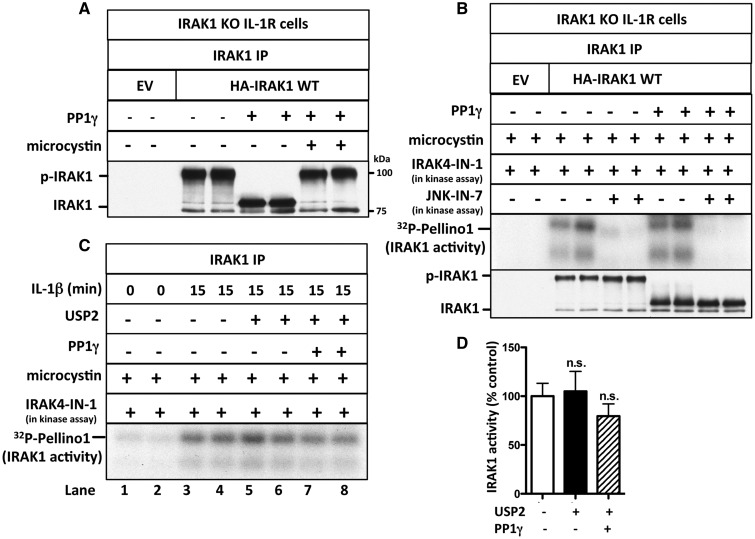
Figure 5. IRAK1 activation does not require its phosphorylation or ubiquitylation.
(A) IRAK1 KO IL-1R cells were transfected with 5 µg of DNA of control empty vector (EV) or HA-IRAK1, and IRAK1 then immunoprecipitated from 0.5 mg of cell extract protein. The IPs were incubated with PP1γ (10 U) in the presence or absence of microcystin (10 µM) and denatured in SDS. Following SDS–PAGE and transfer to PVDF membranes, immunoblotting was performed with anti-IRAK1. (B) As in A, except that, after PP1γ treatment, the immunoprecipitates were washed, incubated for 1 h with 10 µM microcystin, and IRAK1 was assayed with GST-Pellino1 and Mg[γ32P-ATP] as substrates in the absence (−) or presence (+) of JNK-IN-7 (1 µM) and in the presence (+) of IRAK4-IN-1 (1 µM) to inactivate co-immunoprecipitating IRAK4. The presence of IRAK1 in the immunoprecipitates was also analyzed by immunoblotting. (C) IL-1R cells were stimulated with IL-1β and IRAK1 immunoprecipitated from 1 mg of cell extract protein and incubated with PP1γ (10 U) and USP2 (1.15 µg). The immunoprecipitates were washed, incubated for 1 h with 1 µM microcystin to inactivate any residual PP1γ, and IRAK1 assayed with GST-Pellino1 and Mg[γ32P-ATP] as substrates in the presence (+) of IRAK4-IN-1 (1 µM) to inactivate any co-immunoprecipitating IRAK4. (D) The autoradiogram from C and one other independent experiment were scanned and IRAK1 activity quantitated after incubation with or without USP2 and PP1γ. The results are presented as a % of that measured without USP2 and PP1γ treatment.