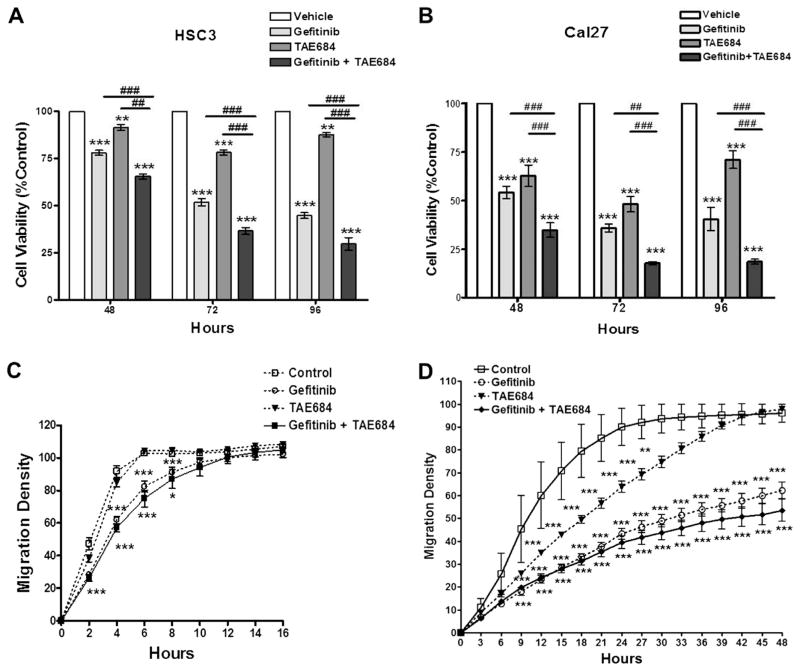
Fig. 2.
Panel A Cell viability assay of HSC3 cells treated with Gefitinib (500 nM), TAE684 (500 nM), or both. Panel B: Cell viability assay of Cal27 cells treated with Gefitinib (500 nM), TAE684 (500 nM), or both. Panel C: Wound-healing assay of HSC3 cells treated with Gefitinib (500 nM), TAE684 (500 nM), or both. Panel D: Wound-healing assay of Cal27 cells treated with Gefitinib (500 nM), TAE684 (500 nM), or both. *p < 0.05, **p < 0.01, ***p < 0.001 treatment compared to control. #p < 0.05, ##p < 0.01, ###p < 0.001 Gefitinib compared to combination treatment. This data represents four replicates and error bars indicate SD.