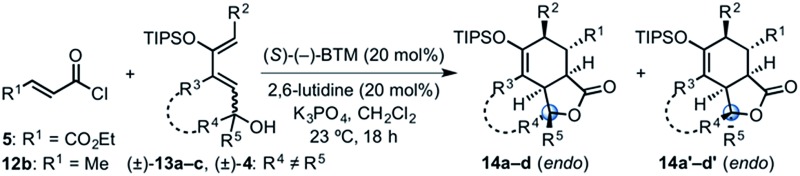
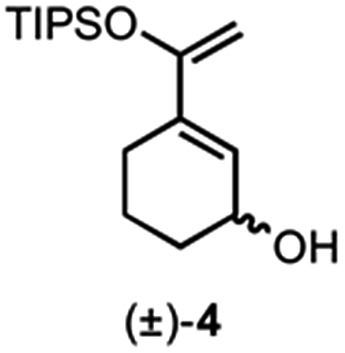
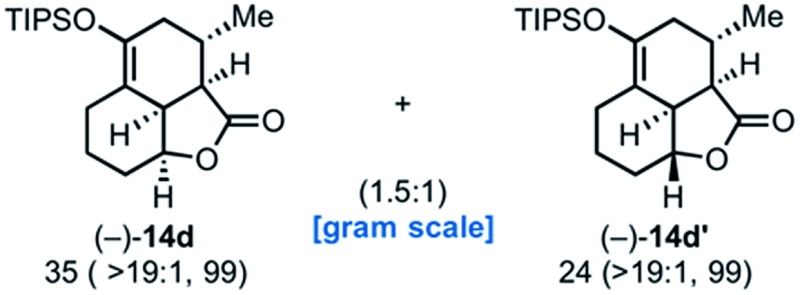
Table 1. Diels–Alder-initiated, stereodivergent organocascades with racemic dienes (±)-13a–d providing bi- and tricyclic γ-lactones 14a–d a .
| |||
| Entry | Diene | Acid chloride | Cycloadducts % yield (endo : exo, % ee) |
| 1 |
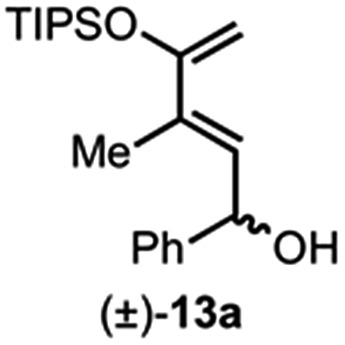
|
12a |
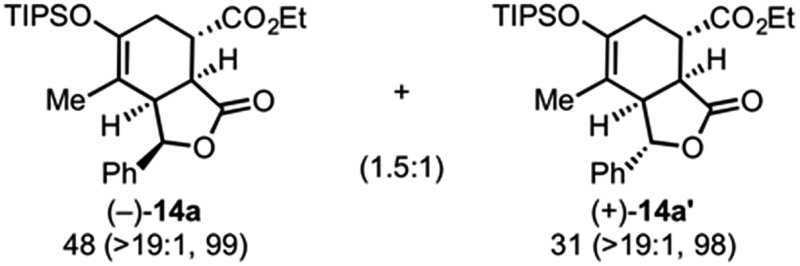
|
| 2 |
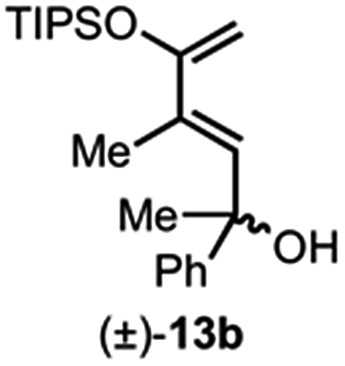
|
12a |
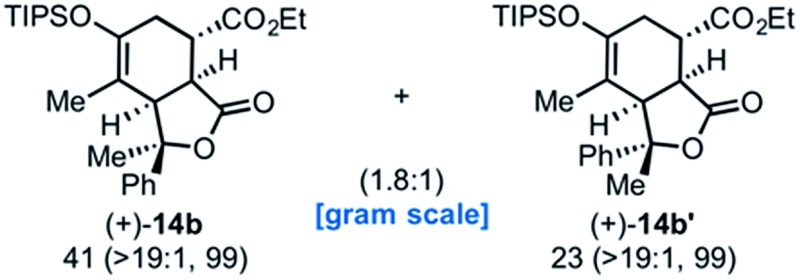
|
| 3 |
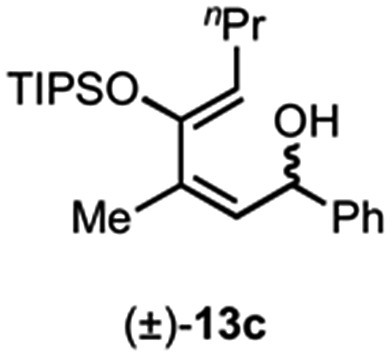
|
12a |
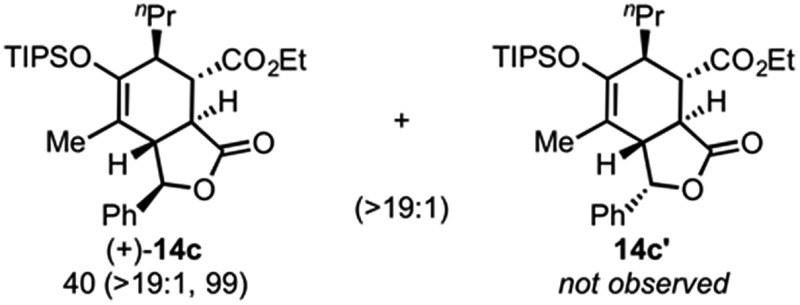
|
| 4 |
|
12b |
|
aUnless otherwise specified, all reactions were performed with diene (1.0 equiv.), acid chloride (1.5 equiv.), K3PO4 (3.0 equiv.), 2,6-lutidine (20 mol%), and (S)-(–)-BTM (20 mol%) at 23 °C for 18 h. Yields are based on isolated, purified cycloadducts. Enantiomeric excess was determined by chiral phase HPLC.
