Table 3. Tunable diastereoselectivity in accessing a stereoisomeric family of bicyclic-γ-lactones through chiral Lewis base catalyst and Brønsted base permutations in the DAL organocascade.
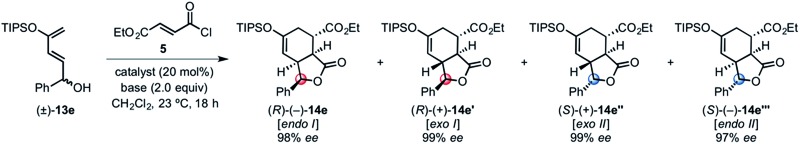
| ||||||
| Entry | Catalyst | Base | (R)-(–)-14e a (%) | (R)-(+)-14e′ a (%) | (S)-(+)-14e′′ a (%) | (S)-(–)-14e′′′ a (%) |
| 1 | A | I | 27 | 22 | 25 | 18 |
| 2 | A | II | 24 | — | 23 | 15 |
| 3 | A | III | 27 | — | — | 21 |
| 4 | A | IV | 29 | — | 20 | — |
| 5 | B | V | — | — | 26 | 19 |
| 6 | B | IV | — | — | 18 | — |
| 7 | C | IV | 22 | — | — | — |
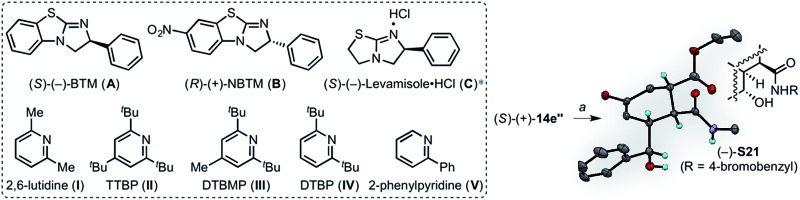
| ||||||
aYields and diastereomeric ratios are based on isolated, purified cycloadducts. Enantiomeric excess was determined by chiral phase-HPLC. *Employed in free-base form. Inset is a single crystal X-ray structure in ORTEP format of adduct (R)-(+)-14′′ following ring opening with 4-bromobenzylamine (50% probability; TIPS and 4-bromobenzyl groups are removed for clarity, see ESI Fig. S1). a4-BrC6H4CH2NH2, THF, 23 °C, 36 h (46%).
