Table 1. Optimized reaction conditions a .
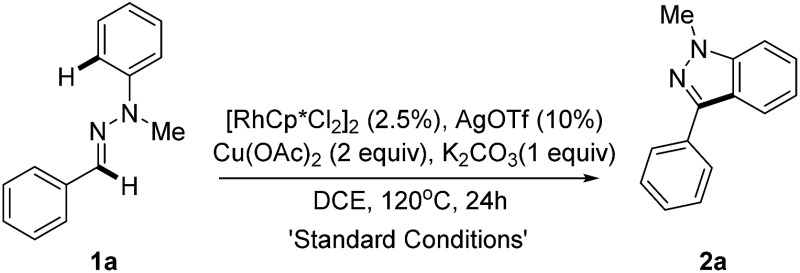
| |||
| Entry | Variation from the “standard conditions” | Conv. of 1a b (%) | Yield b (%) |
| 1 | None | 84 | 81(80) c |
| 2 | Without K2CO3 | 5 | Trace |
| 3 | Without [RhCp*Cl2]2 | 0 | 0 |
| 4 | Without AgOTf | 55 | 50 |
| 5 | 1.5 equiv. of Cu(OAc)2 | 50 | 42 |
| 6 | O2 and without Cu(OAc)2 | 5 | 0 |
| 7 | 135 °C instead of 120 °C | 85 | 80 |
| 8 | 100 °C instead of 120 °C | 66 | 65 |
a The reactions were run on a 0.20 mmol scale in 0.5 mL of DCE.
b Yields determined by 1NMR spectroscopy using N-(4-methoxyphenyl)acetamide as the internal standard.
c Yield of isolated products. Cp* = 1,2,3,4,5-pentamethylcyclopentadiene, and DCE = 1,2-dichloroethane.
