Abstract
Botulism had mortality rates >60% before the 1950s. We reviewed confirmed botulism cases in the USA during 1975–2009 including infant, foodborne, wound, and other/unknown acquisition categories, and calculated mortality ratios. We created a multivariate logistic regression model for non-infant cases (foodborne, wound, and other/unknown). Overall mortality was 3.0% with 109 botulism-related deaths among 3,618 botulism cases [18 (<1%) deaths among 2,352 infant botulism cases, 61 (7.1%) deaths among 854 foodborne botulism cases, 18 (5.0%) deaths among 359 wound botulism cases, and 12 (22.6%) deaths among 53 other/unknown botulism cases]. Mortality among all cases increased with age; it was lowest among infants (0.8%) and highest among persons ≥80 years old (34.4%). Toxin type F had higher mortality (13.8%) than types A, B, or E (range, 1.4% to 4.1%). Efforts to reduce botulism mortality should target non-infant transmission categories and older adults.
Keywords: botulism, mortality, infant, age groups, USA, food
1 Introduction
Botulism is a severe illness that is caused by toxin-mediated blockade of acetylcholine release across the neuromuscular junction. The resulting flaccid paralysis can be fatal when the respiratory muscles are involved. Indeed, >60% of US botulism cases were fatal before the 1950s, though the case-fatality ratio gradually declined to <10% by the 1990s due to increasing availability of positive pressure mechanical ventilation, botulinum antitoxin, and other aspects of intensive supportive care (Centers for Disease Control and Prevention, 1998; Puri et al., 2009); intravenous botulism immune globulin for infants became available in 2003. Though rare, botulism is a medical and public health emergency because of its lethality and the possibility that a single non-infant case indicates a larger outbreak; all states require immediate reporting of suspect cases. The Centers for Disease Control and Prevention (CDC) and the California Department of Health Services (CDHS) provide national 24-hour clinical consultation and antitoxin release service for suspected non-infant and infant cases, respectively1.
Seven serologically distinct botulinum toxin types (A–G) are known. Human botulism is caused primarily by toxin types A, B, or E, and rarely by F produced by C. botulinum. Several related clostridial species can produce botulinum toxins as well; toxin type E can be produced by C. butyricum and type F can be produced by C. baratii. Although most strains produce a single toxin type, rare mixed toxin type strains have been identified that produce two types of toxin (i.e., Ba, Ab, Bf, and Af, with capital letter denoting predominant toxin type).
CDC categorises human botulism cases into four acquisition categories: foodborne, which results from the consumption of foods containing pre-formed botulinum toxin; wound, which occurs when C. botulinum spores germinate under anaerobic conditions within a wound and produce toxin; infant intestinal colonisation (hereafter referred to as infant botulism), which occurs when ingested C. botulinum spores colonise the intestine, germinate, multiply and produce toxin in children aged <1 year; and other/unknown, which includes cases in persons aged ≥1 year for whom a thorough investigation reveals no suspected exposure to contaminated food and who has no wounds. The other/unknown category includes cases of adult intestinal colonisation botulism (caused by intestinal colonisation with in-vivo toxin production by C. botulinum), iatrogenic botulism (caused by an accidental overdose of therapeutic botulinum toxin), as well as laboratory confirmed botulism cases with no known cause. Although foodborne and wound botulism can occur in children aged <1 year, almost all cases in this age group occur through intestinal colonisation (Armada et al., 2003). Previous epidemiologic investigations of botulism have linked higher mortality to age ≥60 years, type A or E versus type B toxin and non-infant acquisition categories, but these studies did not assess or control for the interrelationships of these variables (Hughes et al., 1981; Woodruff et al., 1992; Centers for Disease Control and Prevention, 1998). CDC botulism surveillance data was analysed to describe mortality trends and factors associated with botulism mortality since 1975 and to identify the patient groups that might benefit from specific, targeted efforts to further reduce mortality.
2 Methods
States report to CDC demographic information (e.g., age and gender), date of onset, acquisition category, toxin type and outcome for all cases meeting the Council for State and Territorial Epidemiologists’ (CSTE) botulism case definition (CSTE, 2010). Foodborne, wound, infant and other/unknown acquisition categories were categorised by CDC using the CSTE botulism case definitions (CSTE, 2010; Centers for Disease Control and Prevention, 2012). Adult intestinal colonisation botulism and iatrogenic botulism were included in the ‘other/unknown’ botulism category because the small numbers of cases in each category precluded in-depth analysis and for consistency with the CSTE case definition. For this analysis, non-infant botulism cases were those classified as foodborne, wound, or other/unknown. Six cases of botulism occurring in persons <1 year of age were categorised as foodborne botulism due to evidence indicating that these cases were foodborne. Toxin type was determined through laboratory testing at state public health laboratories and/or CDC via mouse bioassay of serum, stool, wound and/or food specimens (Hatheway, 1988). Mixed and unknown toxin types were excluded from analyses by toxin type. Mixed toxin types were excluded from analyses because there were just 16 cases. Unknown toxin types can occur when clinical specimens are of insufficient quantity. This prevents toxin typing via the mouse bioassay, although botulinum toxicity can still be demonstrated by setting up a limited mouse bioassay with a control mouse and a mouse injected with anti-ABE.
Reports received by CDC of cases meeting the CSTE botulism case definition (CSTE, 2010) in residents of the USA with onset from 1 January 1975, through 31 December 2009 were reviewed. The USA was divided into the following census regions: Midwest (Illinois, Iowa, Indiana, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, Wisconsin and South Dakota), Northeast (Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island and Vermont), South (Alabama, Arkansas, District of Columbia, Delaware, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia and West Virginia) and West (Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington and Wyoming) (United States Census Bureau, http://www.census.gov/). The 35-year study period was divided into seven five-year time periods for the purpose of examining changes over time. The data represent all cases occurring in the surveillance population, not a sample of cases, so tests of statistical significance of changes in mortality ratios by acquisition category over time were not applied. Wound botulism was not analysed by time until 1995 because of low numbers of reported wound botulism prior to 1994 (Werner et al., 2000). Due to the very small number of cases categorised as other/unknown acquisition, this category was not analysed by time.
Mortality ratios were calculated by dividing the number of deaths by the total number of cases with outcome information, overall and within categories of age, gender, acquisition category, toxin type, region, and time period. The associations of these categories with mortality were measured by calculating odds ratios. Mortality by time period stratified by infant and non-infant botulism was calculated. To measure the strength of association of one variable on mortality while accounting for the effects of other variables (i.e., the effect of age adjusting for acquisition category, toxin type and time period), adjusted odds ratios were calculated with a multivariate logistic regression model using variables that were significantly associated (p <0.05) with mortality in bivariate analysis. The final model excludes all cases in persons <1 year of age (both infant botulism and the six cases of non-infant botulism), because the very low mortality in infants obscured the differences between non-infant acquisition categories in a preliminary model; as a result, the model compares mortality between foodborne, wound and other/unknown acquisition categories. Age categories were further collapsed in the multivariate model based on results of the bivariate analysis. Region was excluded from the model both because toxin type is strongly related to geographic region and because nearly all wound botulism occurs in western states (Gangarosa et al., 1971; Werner et al., 2000; Sobel et al., 2004). Records with missing data were excluded for analyses of the variable(s) that were missing.
3 Results
During 1975–2009, botulism-related death was reported in 109 (3.0%) of 3,618 botulism cases in the USA (Table 1); 435 cases without mortality outcome information and an additional 66 cases with unknown or mixed toxin type were excluded from this total and subsequent analyses. Of the reported cases, 2,352 (65.0%) were infant botulism, 854 (23.6%) were foodborne botulism, 359 (9.9%) were wound botulism and 53 (1.5%) were in the other/unknown category (Table 1). Among the cases of foodborne botulism, 561 (65.7%) were associated with an outbreak of two or more persons. Among the few cases of other/unknown botulism, 32 were type A (60.4%), 14 were type F (26.4%) and seven were type B (13.2%). The other/unknown category accounted for nearly half (48.3%) of all type F cases. Most (38, 75%) were reported as botulism of unknown source, ten were reported as suspected adult intestinal colonisation botulism (age range 5–83, median 57.5 years; 50% toxin type A, 30% toxin type F and 20% toxin type B) and five were reported as iatrogenic botulism (age range 34–89, median 52 years; 100% toxin type A).
Table 1.
Botulism mortality by demographic subgroups, acquisition category, toxin type, region and time period, toxin types A, B, E and F, USA 1975–2009 (n = 3,618)*
| Died | Survived | Percent mortality | OR (95% CI) | |
|---|---|---|---|---|
| Age (years) | ||||
| <1 | 17 | 2,327 | 0.7 | Referent |
| 1–9 | 3 | 40 | 7.0 | 10.3 (2.9–36.4) |
| 10–19 | 2 | 56 | 3.4 | 4.9 (1.1–21.7) |
| 20–29 | 6 | 154 | 3.6 | 5.3 (2.1–13.7) |
| 30–39 | 8 | 245 | 3.2 | 4.5 (1.91–10.5) |
| 40–49 | 11 | 262 | 4.0 | 5.8(2.7–12.4) |
| 50–59 | 23 | 200 | 10.3 | 15.4 (7.2–33.0) |
| 60–69 | 12 | 107 | 10.1 | 15.4 (7.2–33.0) |
| 70–79 | 15 | 58 | 20.6 | 35.4 (16.9–74.3) |
| ≥80 | 11 | 21 | 34.4 | 71.7 (30.0–171.4) |
| Unknown | 1 | 39 | 2.5 | 4.4 (0.6–34.2) |
| Gender | ||||
| Male | 59 | 1,805 | 3.2 | Referent |
| Female | 50 | 1,696 | 2.9 | 0.9 (0.6–1.3) |
| Unknown | 0 | 8 | 0.0 | 0.8 (0.0–15.1) |
| Acquisition category | ||||
| Infant | 18 | 2,334 | 0.8 | Referent |
| Foodborne | 61 | 793 | 7.1 | 10.0 (5.9–17.0) |
| Wound | 18 | 341 | 5.0 | 6.9 (3.5–13.3) |
| Other/unknown | 12 | 41 | 22.6 | 38.0 (17.2–83.9) |
| Toxin type | ||||
| F | 4 | 25 | 13.8 | Referent |
| A | 77 | 1,826 | 4.1 | 0.3 (0.1–0.8) |
| B | 20 | 1,437 | 1.4 | 0.1 (0.0–0.3) |
| E | 8 | 221 | 3.5 | 0.2 (0.1–0.8) |
| Region** | ||||
| Midwest | 12 | 272 | 4.2 | Referent |
| Northeast | 8 | 493 | 1.6 | 0.4 (0.2–0.9) |
| South | 19 | 510 | 3.6 | 0.8 (0.4–1.8) |
| West | 69 | 2,232 | 3.0 | 0.7 (0.4–1.3) |
| Time period | ||||
| 1975–1979 | 24 | 315 | 7.1 | Referent |
| 1980–1984 | 27 | 487 | 5.3 | 0.7 (0.4–1.3) |
| 1985–1989 | 8 | 474 | 1.7 | 0.2 (0.1–0.5) |
| 1990–1994 | 9 | 447 | 2.0 | 0.3 (0.1–0.6) |
| 1995–1999 | 10 | 570 | 1.7 | 0.2 (0.1–0.5) |
| 2000–2004 | 12 | 604 | 2.0 | 0.3 (0.1–0.5) |
| 2005–2009 | 19 | 612 | 3.0 | 0.4 (0.2–0.8) |
Notes:
Excludes cases for whom outcome is not known (n = 435) and cases with unknown or mixed toxin type (n = 66),
Excludes three cases from Puerto Rico; n = 3,615
The bivariate analysis in Table 1 reports associations between each variable and mortality, independent of other variables. Botulism mortality generally increased with age and was highest among persons ≥80 years (0.7% vs. 34.4%, respectively; OR 45.05; 95% CI 23.49–86.39) for comparisons to <1 year age group. Males and females had similar mortality (3.2% vs. 2.9%, respectively), even when stratified by acquisition category (data not shown). All non-infant acquisition categories had significantly higher mortality than the infant botulism category (0.8%); mortality was highest among cases classified as other/unknown (22.6%; OR 38.0; 95% CI 17.2–83.9), followed by foodborne (7.1%; OR 10.0; 95% CI 5.9–17.0) and wound (5.0%; OR 6.9; 95% CI 3.5–13.3). Among the other/unknown cases, one death occurred in an adult intestinal colonisation patient (toxin type A) and none occurred in iatrogenic botulism patients; 11 deaths among cases of unknown acquisition category were reported. Mortality among foodborne botulism patients that were associated with an outbreak of two or more persons was lower than among patients that occurred as a single occurrence (5.4% vs. 10.4%, OR 0.5; 95% CI 0.30–0.84). When compared with cases of toxin type F botulism (13.8%), persons with type A (4.1%; OR 0.3; 95% CI 0.1–0.8), type B (1.4%; OR 0.1; 95% CI 0.0–0.3) and type E (3.5%; OR 0.2; 95% CI 0.1–0.8) had significantly lower mortality. Mortality in the Northeast was significantly lower than in the Midwest (1.6% vs. 4.2%, respectively; OR 0.4; 95% CI 0.2–0.9). Mortality decreased over time and was significantly lower than in 1975–1979 in all time periods from 1985–1989 onward. However, crude mortality (excluding cases with unknown or mixed toxin type) among wound and foodborne cases – and all non-infant cases as a group – increased during the last two time periods (Figure 1).
Figure 1. Botulism mortality (deaths per 100 cases) by time period and acquisition category, USA, 1975–2009 (n = 3,618)*, **.
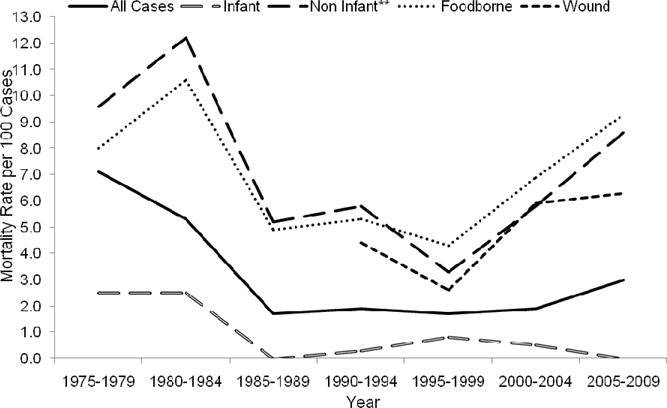
Notes: *Excludes cases with unknown outcome (n = 435) and cases with unknown or mixed toxin type (n = 66),
**Due to the very small number of cases categorised as other/unknown (n = 53), the other/unknown acquisition category was not analysed by time period
***The non-infant category includes all botulism cases classifed as foodborne, wound and other/unknown.
The results of the multivariate logistic regression analysis are shown in Table 2. This analysis included 1,260 cases, as it excludes cases with unknown outcome, mixed or unknown toxin types, infant botulism cases and six cases of foodborne botulism that occurred in persons <1 year. Mortality increased with age and was highest among persons ≥80 (34.4%, vs. 3.8% in 1–49 year-old group; adjusted OR = 17.1; 95% CI 6.9–45.2) when adjusting for the effects of acquisition category, toxin type and time period on mortality. Foodborne botulism was less likely than other/unknown forms to result in death (adjusted OR = 0.3; 95% CI 0.1–0.8) when adjusting for the effects of age group, toxin type and time period on mortality. Wound botulism also had lower, though not statistically significantly lower, mortality than botulism transmitted by other/unknown mechanism (adjusted OR = 0.4; 95% CI 0.2–1.2) when adjusting for the effects of age group, toxin type and time period on mortality. There were no significant differences in mortality by toxin type when adjusting for the effects of age group, acquisition category and time period on mortality. Mortality was lower during 1995–1999 and 2000–2004 than during 1975–1984 (3.3% vs. 9.6%, respectively; adjusted OR = 0.2; 95% CI 0.1–0.7 and 5.8% vs. 9.6%, respectively; adjusted OR = 0.3; 95% CI 0.1–0.7; respectively) when adjusting for the effects of age group, acquisition category and toxin type on mortality.
Table 2.
Multivariate logistic regression analysis of botulism mortality among persons ≥1 year of age, toxin types A, B, E and F, USA 1975–2009*
| Died | Survived | Percent mortality | Adjusted OR (95% CI)** | |
|---|---|---|---|---|
| Age (years) | ||||
| 1–49*** | 29 | 744 | 3.8 | Referent |
| 50–69 | 35 | 307 | 10.2 | 3.2 (1.9–5.5) |
| 70–79 | 15 | 58 | 20.6 | 7.5 (3.5–15.9) |
| ≥80 | 11 | 21 | 34.4 | 17.1 (6.9–45.2) |
| Acquisition category | ||||
| Other/unknown | 12 | 41 | 22.6 | Referent |
| Foodborne | 61 | 787 | 7.2 | 0.3 (0.1–0.8) |
| Wound | 18 | 341 | 5.0 | 0.4 (0.2–1.2) |
| Toxin type | ||||
| F | 3 | 18 | 14.3 | Referent |
| A | 68 | 757 | 8.2 | 1.6 (0.3–7.3) |
| B | 12 | 175 | 6.4 | 1.0 (0.2–5.3) |
| E | 8 | 219 | 3.5 | 0.7 (0.1–3.8) |
| Time period | ||||
| 1975–1979 | 21 | 193 | 9.8 | Referent |
| 1980–1984 | 18 | 130 | 12.2 | 1.4 (0.6–2.8) |
| 1985–1989 | 8 | 147 | 5.2 | 0.5 (0.2–1.3) |
| 1990–1994 | 8 | 130 | 5.8 | 0.5 (0.2–1.2) |
| 1995–1999 | 7 | 205 | 3.3 | 0.2 (0.1–0.7) |
| 2000–2004 | 10 | 161 | 5.9 | 0.3 (0.1–0.7) |
| 2005–2009 | 19 | 203 | 8.6 | 0.5 (0.2–1.1) |
Notes:
Excludes cases for whom outcome is not known (n = 435), cases with unknown or mixed toxin type (n = 66), infant botulism acquisition category (n = 2,352) and foodborne cases that occurred in persons <1 year (n = 6),
The adjusted OR measures the effect between a predictor variable (i.e., age) and mortality while adjusting for other predictor variables (i.e., acquisition category, toxin type and time period),
Does not equal the sum of age groups 1–9…40–49 because 14 infant botulism cases were reported to CDC as occurring in one year olds; all 14 cases occurred in infants aged 63 weeks or younger (median 53 weeks)
4 Discussion
This large study of botulism mortality, the first to include comparisons of all major acquisition categories and the four toxin types that cause human illness, reveals important differences in mortality risk related to the mechanism of acquisition and patient age. With increasing diagnosis of infant botulism, for which mortality has always been very low, US botulism mortality decreased during 1975–2009, with an overall mortality of 3.0 per 100 cases. However, these summary results mask important differences in mortality between acquisition categories. Mortality for non-infant botulism was consistently higher than infant mortality and, though it was lower in 1995–2004 than in 1975–1979, it increased somewhat during the last decade of the study. The recent increase appears to be due to an increasing proportion of cases in older adults, in whom mortality is quite high. Mortality approached 35% among persons aged ≥80 years and age was strongly and independently associated with mortality, even when excluding infants; similar patterns have been reported previously (Tacket et al., 1984; Fagan et al., 2011b). Therefore, efforts aimed at reducing botulism mortality should specifically target non-infant acquisition categories and older patients.
Among non-infant acquisition categories, other/unknown cases had the highest mortality. The other/unknown categorisation includes intestinal colonisation patients, who generally lack typical epidemiologic clues to botulism and in whom; therefore, diagnosis and treatment may be delayed (Fagan et al., 2011a; Filozov et al., 2012; Sheppard et al., 2012). The other/unknown category includes a higher percentage of type F botulism cases than other categories (Gupta et al., 2005). Some of the cases/deaths in the other/unknown acquisition category may have been due to unrecognised adult intestinal colonisation. Heptavalent botulinum antitoxin, the only botulinum antitoxin effective for treating type F botulism, was not available as first-line treatment for non-infant cases until March 2010, which may also contribute to the higher overall mortality in type F patients. Although it may be presumed that patients with iatrogenic botulism would be under ongoing medical supervision and therefore at lower risk of death, four of the five iatrogenic cases included in the other/unknown category received injections of a highly concentrated, unlicensed preparation of botulinum toxin A; although it is estimated that these patients may have received doses 2,857 times the estimated lethal injected dose (Chertow et al., 2006), none of the patients died.
In this study, mortality was significantly associated with toxin type in bivariate analyses only and was highest in types A and F botulism. Type A disease has been previously reported to be more severe than type B or E disease (Hughes et al., 1981; Woodruff et al., 1992) and type F cases have been described as following a characteristically fulminant course with rapid progression to respiratory failure and paralysis (Gupta et al., 2005). Our results indicate that the observed mortality differences among toxin types could be influenced by other factors such as age or acquisition category, which itself implies certain characteristics of the at-risk population. For instance, type F botulism is thought to typically occur as intestinal colonisation among adults with pre-existing gastrointestinal pathology or recent antimicrobial use, which raises the possibility that the relatively severe illness described in these patients is reflective of poorer underlying health status than other groups of botulism patients. Still, our results should not be interpreted as excluding actual syndromic differences among toxin types on the basis of differences in their molecular targets or half-lives (Simpson, 2004).
Prompt administration of botulinum antitoxin has been shown to decrease mortality (Tacket et al., 1984). For two reasons, we were unable to confirm these results. First, although complete data on antitoxin release are available, information about actual administration of antitoxin was rarely available for cases in the early years of the study. Second, even when information on antitoxin administration was available, the time between symptom onset and administration was usually not recorded.
The data on mortality were complete for almost 90% of cases; this low rate of missing data lends credence to the results showing strong association of older age and acquisition category with mortality. Ethnicity and race were not included in the analysis because these data were missing for most cases. Because suspect botulism cases constitute both a medical and public health emergency and because confirmatory testing and specific treatment for suspect botulism patients can only be obtained through public health agencies, our surveillance database should include all or almost all diagnosed cases. However, mild cases likely occurred that did not result in a visit to a physician and diagnoses were likely missed in both mild and severe cases; following the detection of one large foodborne outbreak, 36 previously unrecognised cases were identified; these patients had been misdiagnosed with myasthenia gravis, psychiatric disorders, stroke and others (St. Louis et al., 1988). The inclusion of cases involved in foodborne outbreaks of two or more persons in this analysis may lead to an underestimate of the mortality rate of foodborne botulism as compared to other botulism acquisition categories, as the definition of foodborne botulism includes persons with clinically compatible cases who ate the same food as a laboratory-confirmed case, but whose illnesses may not have been laboratory-confirmed (i.e., milder cases). The inclusion of milder cases may help explain why the mortality among foodborne cases that occur in an outbreak of two or more persons is lower than those that occur as an isolated case. Alternatively, clustered cases are more rapidly diagnosed as botulism if more than one patient is concurrently hospitalised with symptoms consistent with botulism, which could lead to earlier treatment (Newkirk and Hedberg, 2012). Deaths occurring long after the initial diagnosis may not be captured by the surveillance system.
The findings described in the multivariate model, such as increased mortality with age and lower mortality due to foodborne botulism are likely broadly generalisable to other countries. However, mortality rates may vary by country depending on the availability of supportive care and antitoxin, as well as variation in the predominant acquisition category/ies of botulism.
Additional investigation is warranted to understand the associations of age and acquisition category with botulism mortality. A possible explanation for higher mortality among older patients might be the increased presence of underlying comorbidities that could adversely affect survival; however additional information about comorbidities of botulism patients is needed to further evaluate this hypothesis. Our data also lacked detailed information about duration of illness at which death occurred, or contributing factors to botulism deaths (e.g., ventilator-associated pneumonia) that support additional hypothesis generation regarding our findings. Collection of more complete clinical information, which is now mandated by FDA as part of the pre-licensure evaluation of investigational heptavalent botulinum antitoxin, could help address these knowledge gaps (Centers for Disease Control and Prevention, 2010). Efforts to prevent the occurrence of botulism may include targeted education about safe food practices, particularly among home-canners, persons who prepare and consume Alaska Native foods and older adults. Injection drug use should be discouraged and strategies for harm reduction among injection drug users considered. Training physicians in the early recognition of cases and the need to promptly administer antitoxin is also important to minimise mortality.
Biographies
Kelly A. Jackson received her MPH in Epidemiology of Microbial Diseases from Yale University in New Haven, CT. She is currently a Senior Surveillance Epidemiologist at the Centers for Disease Control and Prevention and has been working with botulism surveillance data for many years.
Barbara E. Mahon received her MD from the University of California, San Francisco, in 1989; completed a residency in paediatrics in 1992; and received her MPH in Epidemiology from the University of California, Berkeley, in 1994. She trained in the Epidemic Intelligence Service at the Centers for Disease Control and Prevention and has since worked in governmental, academic, and industry settings on a wide range of infectious diseases. She is currently the Deputy Chief of the Enteric Diseases Epidemiology Branch at CDC.
John Copeland has been a Statistician at the CDC since 2000. During that time, he has worked with the Immunization Program, the BioSense Surveillance Initiative, and the Division of Foodborne, Waterborne, and Environmental Diseases. He currently works with the Epi Info team as a Statistical Consultant and Software Developer.
Ryan P. Fagan received his MD from the University of Missouri-Columbia in 2001, completed a residency in internal medicine at the University of New Mexico, and completed a fellowship in Infectious Diseases at Tulane University. He trained in CDC’s Epidemic Intelligence Service with the Alaska Section of Epidemiology and spent three years as a medical epidemiologist with CDC’s Enteric Diseases Epidemiology Branch in Atlanta. He is currently a medical epidemiologist with CDC’s Division of Healthcare Quality Promotion.
Footnotes
California and Alaska authorise botulinum antitoxin release for non-infant cases within their respective jurisdictions, independently of CDC.
This paper is a revised and expanded version of a paper entitled ‘Botulism mortality in the United States, 1975–2008’ presented at the Council of State and Territorial Epidemiologists, Pittsburgh, PA, 12 June 2011.
Disclaimer
The findings and conclusions of this study are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Contributor Information
Kelly A. Jackson, Enteric Diseases Epidemiology Branch, Centers for Disease Control and Prevention, 1600 Clifton Road NE, MS D63, Atlanta, GA 30333, USA, Fax: (404)639-2205.
Barbara E. Mahon, Enteric Diseases Epidemiology Branch, Centers for Disease Control and Prevention, 1600 Clifton Road NE, MS D63, Atlanta, GA 30333, USA, Fax: (404)639-2205
John Copeland, Biostatistics and Information Management Office, Centers for Disease Control and Prevention, 2500 Century Boulevard, MS E33, Atlanta, GA 30345, USA, Fax: (404)639-3500.
Ryan P. Fagan, Enteric Diseases Epidemiology Branch, Centers for Disease Control and Prevention, 1600 Clifton Road NE, MS D63, Atlanta, GA 30333, USA, Fax: (404)639-2205
References
- Armada M, Love S, et al. Foodborne botulism in a six-month-old infant caused by home-canned baby food. Annals of Emergency Medicine. 2003;42(2):226–229. doi: 10.1067/mem.2003.259. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Handbook for Epidemiologists, Clinicians, and Laboratory Workers. CDC; Atlanta: 1998. Botulism in the United States, 1899–1996. [online] http://www.cdc.gov/ncidod/dbmd/diseaseinfo/files/botulism_manual.htm (accessed 21 December 2012) [Google Scholar]
- Centers for Disease Control and Prevention. Investigational heptavalent botulinum antitoxin (HBAT) to replace licensed botulinum antitoxin AB and investigational botulinum antitoxin E. Morbitidy and Mortality Weekly Report. 2010;59(10):299. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Nationally Notifiable Diseases Surveillance System (NNDSS): Botulism [online] 2012 http://wwwn.cdc.gov/NNDSS/script/conditionsummary.aspx?CondID=25 (accessed 28 January 2012)
- Chertow D, Tan E, et al. Botulism in four adults following cosmetic injections with an unlicensed, highly concentrated botulinum preparation. The Journal of the American Medical Association. 2006;296(20):2476–2479. doi: 10.1001/jama.296.20.2476. [DOI] [PubMed] [Google Scholar]
- CSTE. Expanding Wound Botulism Surveillance Case Definition [online] 2010 http://www.cste.org/ps2010/10-ID-03.pdf (accessed 26 August 2011).
- Fagan R, Neil K, et al. Initial recovery and rebound of type F intestinal colonization after administration of investigational heptavalent botulinum antitoxin. Clinical Infectious Diseases. 2011a;53(9):125–128. doi: 10.1093/cid/cir550. [DOI] [PubMed] [Google Scholar]
- Fagan RP, McLaughlin JB, et al. Endemic foodborne botulism among Alaska Native persons – Alaska, 1947–2007. Clinical Infectious Diseases. 2011b;52(5):585–592. doi: 10.1093/cid/ciq240. [DOI] [PubMed] [Google Scholar]
- Filozov A, Kattan J, et al. Asymmetric type F botulism with cranial nerve demyelination. Emerging Infectious Diseases. 2012;18(1):102–104. doi: 10.3201/eid1801.110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarosa EJ, Donadio JA, et al. Botulism in the United States, 1899–1969. American Journal of Epidemiology. 1971;93(2):93–101. doi: 10.1093/oxfordjournals.aje.a121239. [DOI] [PubMed] [Google Scholar]
- Gupta A, Sumner C, et al. Adult botulism type F in the United States, 1981–2002. Neurology. 2005;65(11):1694–1700. doi: 10.1212/01.wnl.0000187127.92446.4c. [DOI] [PubMed] [Google Scholar]
- Hatheway C. Botulism. In: Balows A, et al., editors. Laboratory Diagnosis of Infectious Diseases: Principles and Practice. Springer-Verlag; New York: 1988. [Google Scholar]
- Hughes JM, Blumenthal JR, et al. Clinical features of types A and B foodborne botulism. Annals of Internal Medicine. 1981;95(4):442–445. doi: 10.7326/0003-4819-95-4-442. [DOI] [PubMed] [Google Scholar]
- Newkirk R, Hedberg C. Rapid detection of foodborne botulism outbreaks facilitated by epidemiological linking of cases: Implications for food defense and public health response. Foodborne Pathogens and Disease. 2012;9(2):150–155. doi: 10.1089/fpd.2011.0971. [DOI] [PubMed] [Google Scholar]
- Puri N, Puri V, et al. History of technology in the intensive care unit. Critical Care Clinics. 2009;25(1):185–200. doi: 10.1016/j.ccc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Sheppard YD, Middleton D, et al. Intestinal toxemia botulism in 3 adults, Ontario, Canada, 2006–2008. Emerging Infectious Diseases. 2012;18(1):1–6. doi: 10.3201/eid1801.110533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. Identification of the major steps in botulinum toxin action. Annual Review of Pharmacology and Toxicology. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- Sobel J, Tucker N, et al. Foodborne botulism in the United States, 1990–2000. Emerging Infectious Diseases. 2004;10(9):1606–1611. doi: 10.3201/eid1009.030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Louis M, Peck SHS, et al. Botulism from chopped garlic: delayed recognition of a major outbreak. Annals of Internal Medicine. 1988;108(3):363–368. doi: 10.7326/0003-4819-108-3-363. [DOI] [PubMed] [Google Scholar]
- Tacket CO, Shandera WX, et al. Equine antitoxin use and other factors that predict outcome in Type A foodborne botulism. American Journal of Medicine. 1984;76(5):794–798. doi: 10.1016/0002-9343(84)90988-4. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. Census Regions and Divisions of the United States. USCB; Washington DC: [online] http://www.census.gov/geo/maps-data/maps/pdfs/reference/us_regdiv.pdf. [Google Scholar]
- Werner S, Passaro D, et al. Wound botulism in California, 1951–1998: recent epidemic in heroin injectors. Clinical Infectious Diseases. 2000;31(4):1018–1024. doi: 10.1086/318134. [DOI] [PubMed] [Google Scholar]
- Woodruff B, Griffin P, et al. Clinical and laboratory comparison of botulism from toxin types A, B, and E in the United States, 1975–1988. The Journal of Infectious Diseases. 1992;166(6):1281–1286. doi: 10.1093/infdis/166.6.1281. [DOI] [PubMed] [Google Scholar]


