Abstract
We tested the hypothesis that exposure of glut3+/− mice to a ketogenic diet ameliorates autism-like features, which include aberrant behavior and electrographic seizures. We first investigated the life course sex-specific changes in basal plasma–cerebrospinal fluid (CSF)–brain metabolic profile, brain glucose transport/uptake, glucose and monocarboxylate transporter proteins, and adenosine triphosphate (ATP) in the presence or absence of systemic insulin administration. Glut3+/− male but not female mice (5 months of age) displayed reduced CSF glucose/lactate concentrations with no change in brain Glut1, Mct2, glucose uptake or ATP. Exogenous insulin-induced hypoglycemia increased brain glucose uptake in glut3+/− males alone. Higher plasma-CSF ketones (β-hydroxybutyrate) and lower brain Glut3 in females vs males proved protective in the former while enhancing vulnerability in the latter. As a consequence, increased synaptic proteins (neuroligin4 and SAPAP1) with spontaneous excitatory postsynaptic activity subsequently reduced hippocampal glucose content and increased brain amyloid β1-40 deposition in an age-dependent manner in glut3+/− males but not females (4 to 24 months of age). We then explored the protective effect of a ketogenic diet on ultrasonic vocalization, sociability, spatial learning and memory, and electroencephalogram seizures in male mice (7 days to 6 to 8 months of age) alone. A ketogenic diet partially restored sociability without affecting perturbed vocalization, spatial learning and memory, and reduced seizure events. We conclude that (1) sex-specific and age-dependent perturbations underlie the phenotype of glut3+/− mice, and (2) a ketogenic diet ameliorates seizures caused by increased cortical excitation and improves sociability, but fails to rescue vocalization and cognitive deficits in glut3+/− male mice.
Glut3+/− mice expressed age-dependent neurometabolic and behavioral perturbations in males alone, with females protected. A ketogenic diet reduced seizures and partially restored sociability in males.
Glucose is an essential substrate that fuels oxidative metabolism of the brain. Circulating glucose is transported across the blood–brain barrier and into neurons and glial cells by a process of facilitative diffusion (1). This process is mediated by glucose transporters, namely Glut1 isoform expressed in the blood–brain barrier (microvascular endothelium and astrocytes) (2) and Glut3 (neurons) (3). In addition, in select neuronal cells limited anatomically to certain regions of the brain, Glut8 and Glut4 are detected, whereas Glut2 is limited to astrocytes of the hypothalamus (4, 5). More recently Glut6 has been observed to be intracellular in certain neural cells as well (6), although the exact location remains unknown. However, the predominant isoform expressed uniformly by all neuronal cells is Glut3.
We previously determined the biological importance of Glut3 by deleting this gene. Although homozygous null mice suffered early embryonic demise (7), heterozygous null mice survived but developed seizures detected by electroencephalogram (EEG) (8) that converted to clinical seizures under hypoxic-ischemic conditions (9). In addition, these mice exhibited reduced postnatal vocalization upon separation from mothers, perturbed sociability skills, repetitive movements, and aberrant spatial learning and memory reflective of altered cognition (8). Despite these clinical features reminiscent of a neurodevelopmental disorder such as the autism spectrum disorder, there was no change in brain 2-deoxyglucose uptake, although an increase in brain lactate (Lac) uptake was evident in juveniles at 2 months of age (8). This was related to a compensatory increase in the brain Glut1 and monocarboxylate transporter isoform 2 (Mct2) concentrations seen at this early age (8).
Against the backdrop of our prior observations limited to 2 months of age with no sex-specific distinction, we questioned the underlying metabolic derangements encountered in glut3+/− mice at subsequent adult stages along the entire adult life course in a sex-specific manner. To this end, we tested the hypotheses that glut3+/− genotype (1) expresses blood–cerebrospinal fluid (CSF)–brain metabolic changes (glucose, Lac, and/or ketones) responsible for the presenting phenotype at different stages in the adult (spanning 3 to 6 months of age) and during aging (8 to 24 months), and (2) these metabolic changes can be overcome by alternate energy substrates (e.g., ketogenic diet) that would ultimately rescue the clinical phenotype, namely perturbed neurobehavior and EEG seizures. We tested these hypotheses initially in males and females when establishing the baseline neuro-metabolic phenotype. Based on these results, we subsequently focused primarily on males for EEG, cortical synaptic activity, and neurobehavioral studies.
Materials and Methods
Animals
The glut3 (Slc2a3) heterozygous null and wild-type (WT) C57Bl/6 mice were housed in 12-hour light and 12-hour dark cycles and maintained in approved barrier facilities of mouse housing. All mouse studies were conducted as approved by the Animal Research Committee of the University of California, Los Angeles, in accordance with the guidelines established by the National Institutes of Health. Mice were euthanized by intraperitoneal phenobarbital (100 mg/kg) except as noted. Experimental design consisting of studies performed in an age-dependent and sex-specific manner (Supplemental Fig. 1 (4MB, tiff) ).
Baseline adult investigations
Body weight, plasma, and CSF metabolites
Body weight was assessed in 5-month-old male and female mice. CSF was obtained from the cisterna magna compartment in anesthetized mice (under inhalational isoflurane). Blood was obtained from the common carotid artery and the plasma was separated by centrifugation. Brains were collected following craniotomy and immediately snap-frozen in liquid nitrogen and powdered on dry ice. Glucose, Lac, and total ketone concentrations were measured by Liquid Glucose Measurement Set (Pointe Scientific, Lincoln Park, MI), Lactate Assay kit (BioVision, Mountain View, CA) and Autokit total ketone (Wako, Osaka, Japan), respectively, following the manufacturers’ instructions. Brain adenosine triphosphate (ATP) was quantified by the ATP bioluminescence Assay kit HS II (Roche, Indianapolis, IN) following the manufacturer’s instructions.
Tissue glucose transport and uptake
Positron emission tomographic assessment of brain glucose uptake.
WT and glut3+/− mice (2.5 to 3 months old) were injected with ∼25 μCi of 18F-fluorodeoxyglucose (obtained from the University of California Los Angeles Department of Nuclear Medicine) into the tail vein while sedated under inhalational isoflurane. One hour later, the mice were imaged in a G4 positron emission tomography (PET)/X-ray scanner (Sofie Biosciences, Culver City, CA) for 10 minutes followed by a computed tomography scan performed on the CrumpCAT computed tomography scanner (10). Images were visualized and quantified using Amide software (11).
Radiotracer assessment of brain glucose transport/uptake.
Determination of glucose transport rate using 3-O-([3H]-methyl)-d-glucose ([3H]-3-OMG; Sigma, St. Louis, MO) and glucose uptake using deoxy-2-glucose ([14C]-2DG; Perkin Elmer Life Science, Boston, MA) was accomplished by injecting the two tracers into the tail vein (2.5 µCi of each tracer/mouse) of 5-month-old male and female mice. The tissue to plasma concentration ratio was used as an index of tissue (3-O-([3H]-methyl)-d-glucose and deoxy-2-glucose uptake from the blood and was expressed as glucose uptake rate in micromole/gram of tissue/min as previously described (9, 12).
Insulin-induced hypoglycemia on brain 2-deoxyglucose uptake, glucose-6-phophate, and ATP content.
Hypoglycemia was induced by injecting insulin intraperitoneally (Novolin-R; Novo Nordisk, Clayton, NC) at 1 to 2 U/kg that was targeted at decreasing plasma glucose to less than 80 to 90 mg/dL by 90 minutes in 5- to 6-month-old male and female mice. 2-deoxy-(14C)-glucose (1 µCi/g body weight) was administered intraperitoneally 45 minutes after the insulin injection. Mice were euthanized 90 minutes after administration of insulin. The whole brain (cerebrum, cerebellum) was harvested; one-half of the brain was quickly frozen in liquid nitrogen and powdered to assess radioactivity. Brain glucose uptake and glucose-6-phosphate concentrations were calculated and expressed in micromoles per gram of tissue per minute, as previously described (12). The other half of brain was immediately processed for mitochondrial isolation (Mitochondrial Fraction kit; Active Motif, Carlsbad, CA). Mitochondrial ATP was measured using the ATP determination kit (Molecular Probes, Eugene, OR) in nanomole per microgram of protein.
Brain nutrient transporters
Brain Glut1, Glut3, and Mct2 were quantified at 5 months of age in both males and females by Western blot analysis, as previously described (8, 13) (antibodies used are depicted in Supplemental Table 2 (10.8KB, xlsx) ).
Synaptic studies
Synaptic molecular network expression studies.
The synaptic molecules chosen for quantification were based on an established role for each of them in maintaining synaptic integrity, with mutations proving to be disruptive or increased expression of certain molecules observed in the human condition of autism spectrum disorders (14). Cerebral cortex and hippocampus were microscopically dissected separately from whole brains of 4- to 5-month-old male mice and messenger RNAs (mRNAs) quantified, as previously described (15), by using primer sets shown in Supplemental Table 1 (14.2KB, docx) .
Synaptic electrophysiology in cortical slices.
Spontaneous excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs) were recorded in cortical pyramidal neurons (frontal cortex, layers II-III) in glut3+/− and WT male littermates at 2 (juveniles) and 12 months (aging) of age. Mice were deeply anesthetized with isoflurane and decapitated. Preparation of slices and whole-cell patch clamp recordings were obtained following standard procedures (16). After basic membrane properties were measured, spontaneous EPSCs and IPSCs were recorded while holding the membrane potential at −70 and +10 mV, respectively, and in the presence of selective blockers. Frequency and amplitude of spontaneous events were measured using the MiniAnalysis program (Synaptosoft, Fort Lee, NJ) to construct amplitude frequency, cumulative frequency, and amplitude probability histograms.
Baseline aging investigations
Magnetic resonance spectroscopy in vivo studies
Studies were performed on a Bruker Biospin 7-Tesla 30-cm magnetic resonance imaging/magnetic resonance spectroscopy (MRS) system (Bruker Medizintechnik, Ettlingen, Germany). Levels of hippocampal glucose, Lac, γ-aminobutyric acid (GABA), glutamate (Glu), glutamine, and N-acetyl aspartate were measured in vivo using MRS in 8- to 10-month-old male glut3+/− and WT mice. Localized water-suppressed single-voxel 1H MRS (echo time, 20 ms; repetition time, 2500 ms; spectral width, 4000 Hz; 1280 averages, 42 minutes’ acquisition time) was performed using the point-resolved spectroscopy pulse sequence provided by the manufacturer. For each mouse, a 3 × 3 × 2-mm3 voxel was located in the hippocampus based on anatomical landmarks using fast low angle shot imaging. First- and second-order shim terms were automatically adjusted using FASTMAP. A nonwater-suppressed acquisition using eight averages was also acquired and used for absolute quantification. Spectral data were reconstructed with an in-house processing pipeline written in Interactive Data Language software (Harris Geospatial Solutions, Broomfield, CO). Frequency drift during the acquisition time was controlled by collecting the data in 13.5-minute blocks and automatically aligning and summing frequency spectra obtained during each block. The summed spectra were then transformed back to the time domain and analyzed with prior knowledge-based fitting software (LC Model Inc., Oakville, Ontario, Canada) that produced water-referenced measures of absolute brain metabolite concentration. Measurements with Cramer-Rao lower bound values lower than 20% were considered as reliable quantification and were included in the final analysis.
Brain amyloid β protein studies
Brain amyloid β protein concentrations.
Powdered frozen male and female 8- and 24-month-old mouse brains were homogenized and the concentrations of Aβ 1-40 and Aβ 1-42 in protein extracts were quantified by an enzyme-linked immunosorbent assay kit (Wako, Osaka, Japan), as previously described (17).
Brain amyloid β protein immunostaining.
Brain sections were immunostained using a 1:500 dilution of antimouse anti–β-amyloid 1-40 (Covance, Berkeley, CA) and 1-42 (Millipore Corp, Billerica, MA) primary antibodies (Supplemental Table 2 (10.8KB, xlsx) ) in male wild-type and glut3+/− mice, as previously described (3, 18).
Interventional investigation
Ketogenic diet
Male glut3+/− and WT mice were given either a ketogenic diet (protein, 24.8%; fat, 57.3%; fiber, 9.6%; carbohydrate, 0%; 5TJQ, Test Diet, Richmond, IN) or a regular chow diet (protein, 23.4%; fat, 4.5%; fiber, 5.3%; nitrogen-free extract, 49.9%; Omnitreat Tab, 20 mg; Test Diet) during 0 to 21 days of gestation (embryonic/fetal exposure), lactation (exposure in suckling phase, postnatal 1-21), and in the offspring postweaning into adult stages of life until tested. Mice on either a ketogenic or chow diet were both tested simultaneously. At postnatal 7, one set of mice were subjected to ultrasonic vocalization testing; this same set was subjected to sociability testing at 40 days of age. Another set of mice at 3 months of age underwent Morris water maze testing, and after a break of ∼3 to 5 months, some of these same mice were monitored continuously for 24 hours (at 6 to 8 months of age) by video-EEG.
Neurobehavioral testing
Ultrasonic vocalization, sociability testing, and Morris water maze test were carried out in mice, as previously described (8, 17).
Continuous EEG monitoring
Under isoflurane inhalational anesthesia, two leads of the EEG transmitter were implanted on the dura on each side of the frontoparietal cranium, and the frequency of EEG seizures and time spent with EEG evidence of seizures were analyzed, as previously described (8, 9).
Data analyses
All data are expressed as mean ± standard error of the mean. Student t test or Fisher exact test was used for body weight, serum and CSF metabolites, brain protein concentrations, glucose uptake, brain MRS, and seizure investigations. Student t test with Bonferroni correction when indicated or two-way repeated measures analysis of variance was used for neurobehavioral and electrophysiological investigations. When significant differences were seen (P < 0.05), Tukey post hoc analysis was performed to determine intergroup differences. In the case of nonparametric data related to synaptic molecules, Mann-Whitney rank sum test was used. Null hypothesis was rejected at P < 0.05. All statistical analyses were performed using Sigma Stat 3.5 software.
Results
Baseline phenotypic expression
Blood–CSF–brain metabolic profile
Baseline metabolic measurements.
Body weight and plasma glucose were similar between WT and glut3+/− male mice at 5 months of age; however, CSF glucose, plasma Lac, and CSF Lac were significantly lower in glut3+/− than in WT male mice (at 72.3%, 64.1%, and 75.0% vs glut3+/+, respectively; P < 0.05 for each measurement). No differences were detected in either plasma or CSF β-hydroxybutyrate (β-HB) concentrations in glut3+/− vs WT male mice. In contrast, brain ATP concentration in glut3+/− vs WT male mice was 1.5-fold higher in the former genotype (P < 0.05) (Table 1). Body weight, CSF glucose, plasma and CSF Lac, and β-HB along with brain ATP concentrations were not different between glut3+/− and WT female mice. However, plasma glucose was 1.3-fold greater in glut3+/− compared with WT female mice (P < 0.05) (Table 1). Although female glut3+/− plasma glucose concentration mimicked the levels in male glut3+/− and WT concentrations (Table 1), the female WT plasma glucose concentrations were relatively lower (Table 1). Comparison between male and female plasma and CSF β-HB concentrations in both genotypes revealed a 2.5- to 3.5-fold increase in females vs males (in both WT and glut3+/− plasma, P < 0.001; CSF in WT, P < 0.04; glut3+/− trending toward an increase but not significant, P = 0.15) (Table 1).
Table 1.
Baseline Metabolic Phenotypic Changes in 5-Month-Old Male and Female WT and glut3+/− Mice
| Genotype |
Male | Female | ||
|---|---|---|---|---|
| WT (n = 8–12) | glut3+/− (n = 10–14) | WT (n = 9–17) | glut3+/− (n = 9–18) | |
| Body weight (g) | 31.3 ± 2.0 | 30.7 ± 0.9 | 25.1 ± 1.6a | 24.7 ± 1.0 |
| Plasma glucose (mM) | 9.3 ± 0.7 | 9.0 ± 0.5 | 6.7 ± 0.5a | 8.5 ± 0.5b |
| CSF glucose (mM) | 4.7 ± 0.2 | 3.4 ± 0.2c | 4.2 ± 0.3 | 4.3 ± 0.2 |
| Plasma lactate (mM) | 3.9 ± 0.4 | 2.5 ± 0.4c | 2.9 ± 0.2a | 2.9 ± 0.2 |
| CSF lactate (mM) | 2.4 ± 0.3 | 1.8 ± 0.2b | 2.3 ± 0.1 | 2.0 ± 0.1 |
| Plasma β-HB (μM) | 89.7 ± 20.7 | 107.0 ± 24.0 | 367.0 ± 49.0a | 343.0 ± 72.0 |
| CSF β -HB (μM) | 20.4 ± 4.1 | 17.1 ± 2.0 | 79.1 ± 26.5a | 52.8 ± 29.1 |
| Brain ATP (nmol/mg protein) | 16.7 ± 2.1 | 25.5 ± 1.3c | 22.7 ± 3.2 | 28.2 ± 3.2 |
Data are shown as mean ± standard error.
P < 0.01, female WT vs male WT (sex-specific differences).
P < 0.05, glut3+/− vs respective WT (genotype-specific differences).
P < 0.01, glut3+/− vs respective WT (genotype-specific differences).
Brain glucose transport/uptake.
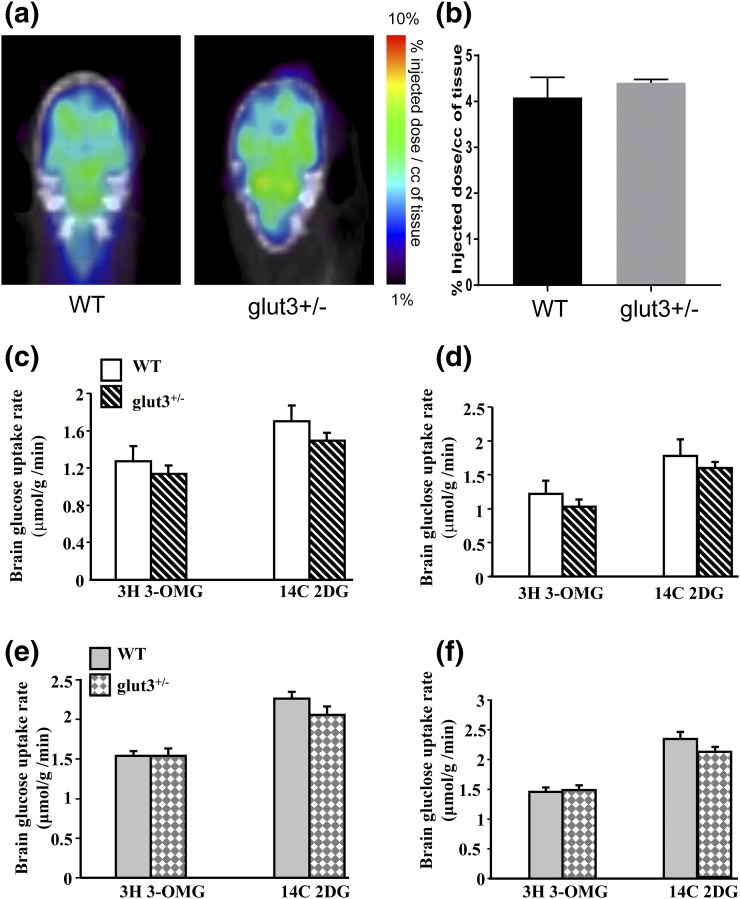
In 2.5- to 3-month-old male mice, no difference in brain 18F-deoxyglucose uptake was observed between wt and glut3+/− genotypes when assessed by PET scanning [Fig. 1(a) and 1(b)]. Additionally, in both 5-month-old males and females, no difference in cerebral and cerebellar 3-OMG (transport) and 2-DG (uptake) was observed [Fig. 1(c–f)]. Similarly, in males the Glut3-expressing testis demonstrated no difference between the two genotypes, neither did any of the other male and female tissues that do not express substantial amounts of Glut3 (heart, kidney, skeletal muscle, lung, liver, and white fat) (data not shown).
Figure 1.
Brain glucose uptake. Positron emission tomography scan images (a) and radiotracer glucose uptake (b) in WT and glut3+/− mouse male cerebrum (c) and cerebellum (d) and female cerebrum (e) and cerebellum (f). *P < 0.05 vs WT. WT mice (male, n = 6; female, n = 6) and glut3+/− mice (male, n = 7; female, n = 7). Glut3-expressing testis (3-0-MG: glut3+/− = 0.3 ± 0.03 vs WT = 0.4 ± 0.08; 2-DG: glut3+/− = 0.4 ± 0.03 vs WT = 0.5 ± 0.07 µmol/g/min) also did not demonstrate a difference between the two genotypes.
Insulin-induced hypoglycemic measurements.
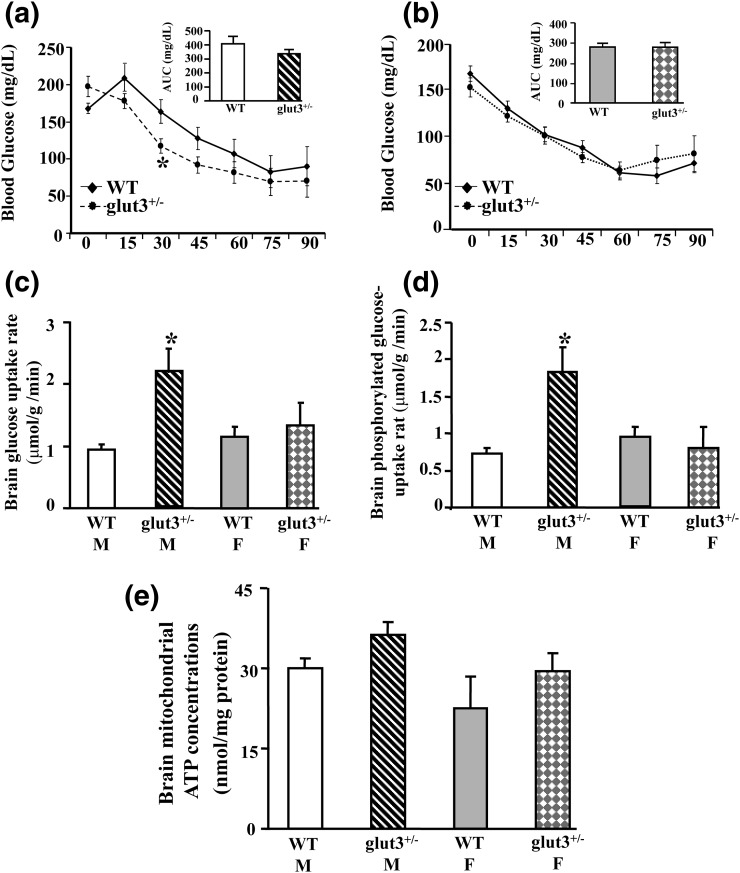
We next perturbed the circulating blood glucose concentration to examine the effect on brain glucose uptake. Although male glut3+/− mice (5 to 6 months) displayed relatively higher baseline glucose concentrations, a greater insulin-induced decrease in glucose concentrations was noted vs the sex-matched WT mice, although not evident in the glucose area under the curve calculations. In contrast, no such difference between the genotypes was evident in female mice. Insulin-induced hypoglycemia was associated with increased whole brain glucose uptake (cerebral and cerebellar) in male but not the female glut3+/− mice when compared with the sex-matched WT counterpart (P < 0.05). Glucose-6-phosphate concentrations followed the same pattern as seen with brain glucose uptake, demonstrating sex-specific differences between glut3+/− and WT genotypes. Whole-brain mitochondrial ATP concentration was not different between the insulin-induced hypoglycemic male and female WT and glut3+/− mice [Fig. 2(a–e)].
Figure 2.
Hyperinsulinemic hypoglycemic studies. Time-dependent changes in blood glucose concentration in (a) male (M) and (b) female (F) WT and glut3+/− mice after administration of exogenous insulin (1 to 2 U/kg). Inset: glucose area under the curve (AUC; mg/dL). (c) Total cerebral 2-deoxy-(14C)-glucose uptake in male and female WT and glut3+/− mice 90 minutes after insulin administration, *P < 0.05 vs male WT mice. (d) Phosphorylated glucose uptake in male and female WT and glut3+/− mice 90 minutes after insulin administration. *P < 0.05 vs male WT mice. (e) Whole-brain mitochondrial ATP concentration in male and female WT and glut3+/− mice 90 minutes after receipt of insulin. WT mice (male, n = 7; female, n = 9) and glut3+/− mice (male, n = 7; female, n = 7).
Brain glucose and monocarboxylate transporters.
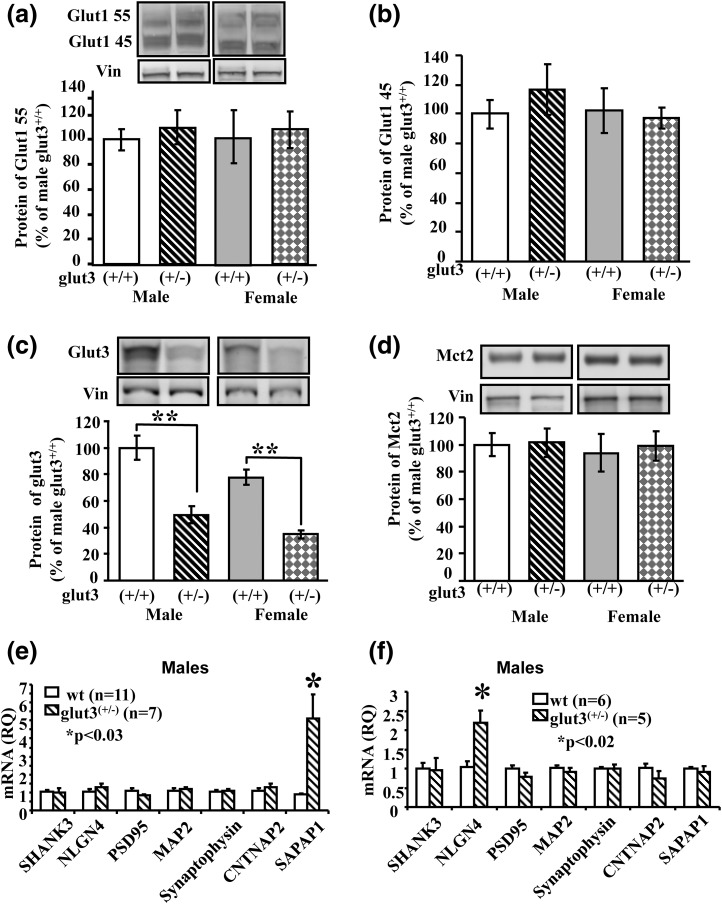
As expected, the glut3+/− male and female mice compared with the WT counterparts exhibited reduced brain Glut3 protein concentration (P < 0.05), with no compensatory increase in either brain Glut1 (both the 55-kDa endothelial cell and 45-kDa glial cell forms) [Fig. 3(a,b)] or neuronal Mct2 (P > 0.05) [Fig. 3(d)]. Comparison of male and female brain Glut3 protein concentrations revealed a 25% reduction in the latter vs the former sex in both genotypes (WT, P < 0.03; glut3+/−, P < 0.017) [Fig. 3(c)]. Neuronal and glial cell-specific isolation studies were attempted but not successful because of contamination by the other cell type (Supplemental Fig. 2 (4.6MB, tiff) ).
Figure 3.
Brain nutrient transporters and genes encoding synaptic proteins. Brain Glut1 55 kDa (a), Glut1 45 kDa (b), Glut3 (c), and Mct2 (d) in WT and glut3+/− male and female mice. Representative western blots for each protein are shown in the top panels (boxes indicate contiguous lanes per sex), and quantification by densitometry is shown in respective bottom panels. n = 10 in each group. **P < 0.01 vs WT mice. (e) Reverse transcription quantitative polymerase chain reaction analysis of cortical SHANK3, NLGN4, PSD95, MAP2, Synaptophysin, CNTNAP2, and SAPAP1 mRNAs in male WT (n = 11) and glut3+/− (n = 7) mice. (f) Real-time quantitative reverse transcription polymerase chain reaction analysis of hippocampal SHANK3, NLGN4, PSD95, MAP2, synaptophysin, CNTNAP2, and SAPAP1 mRNAs in male WT (n = 6) and glut3+/− (n = 5) mice.
Neuronal/synaptic state
Given that metabolic changes were mainly seen in the males, the neuronal and synaptic studies were performed in male mice alone.
Synaptic molecular expression.
No differences were seen between glut3+/− and WT male mice with respect to cerebral cortical [Fig. 3(e)] and hippocampal [Fig. 3(f)] PSD95, SHANK3, MAP2, synaptophysin, and CNTNAP2 mRNAs. However, cerebral cortical SAPAP1 increased in glut3+/− vs WT mice, with no such difference seen in the hippocampal region, whereas hippocampal NLGN4 increased in glut3+/− vs WT mice [Fig. 3(e) and 3(f)]. These results suggest no synaptic molecular disruption despite a reduction in Glut3 protein, although the signaling process (SAPAP1 and NLGN4) may be perturbed to compensate for the relative lack of neuronal Glut3.
Cerebral cortical spontaneous synaptic activity.
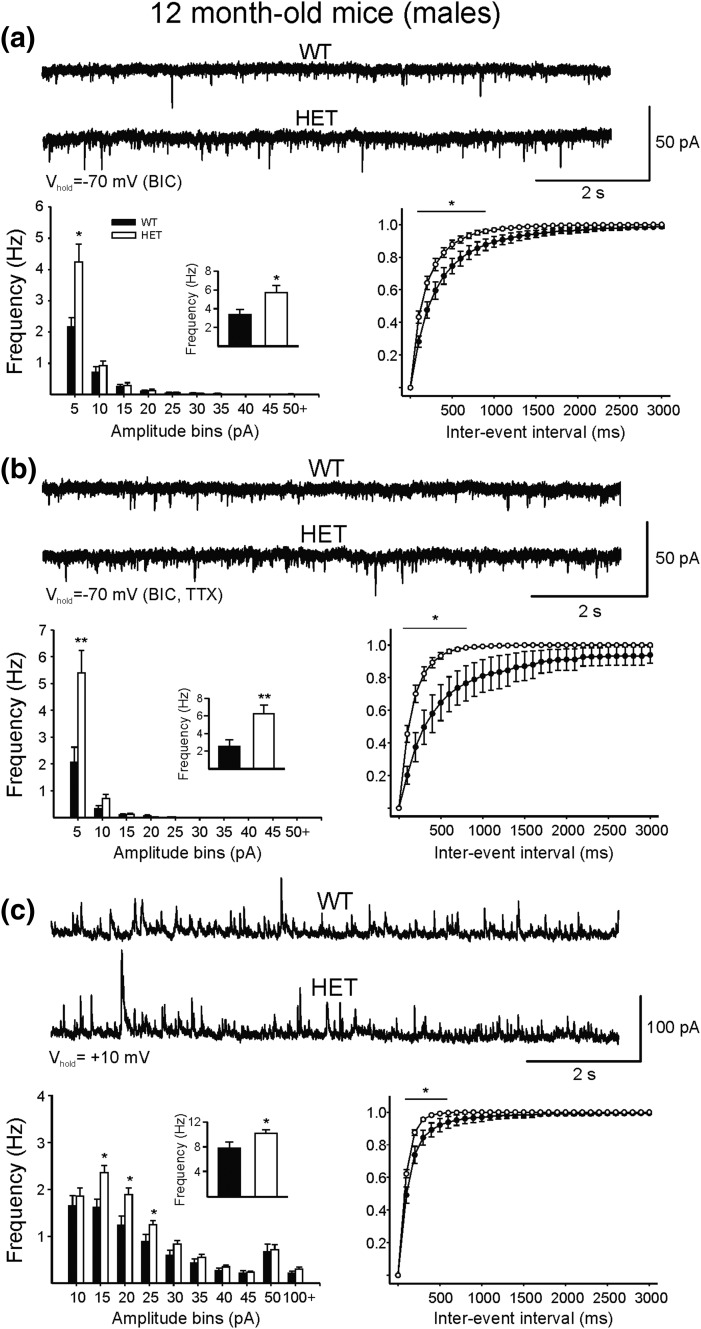
To understand the cause of heightened excitability and seizure susceptibility reported previously in glut3+/− mice (8), recordings of spontaneous synaptic activity from cortical pyramidal neurons were obtained in WT and glut3+/− male mice at 2 months (to match our previous study) (8) and 12 months (at a stage of aging) [Fig. 4(a–c)]. Cortical hyperexcitability occurs either from changes in intrinsic membrane properties or by reduced GABAergic inhibition, increased glutamatergic excitation, or a combination. Cell membrane capacitance, input resistance, and time constant were similar in pyramidal neurons from WT and glut3+/− mice at both ages. At 2 months, no difference in frequency or amplitude of spontaneous excitatory or inhibitory postsynaptic currents (EPSCs and IPSCs) was observed. In contrast, at 12 months, the frequency of spontaneous, Glu receptor-mediated EPSCs was increased [Fig. 4(a)]. Further, after blockade of GABAA receptors with bicuculline, synaptic EPSC bursts and epileptiform discharges occurred more often in glut3+/− mice (data not shown). The increase in frequency of spontaneous EPSCs persisted in the presence of tetrodotoxin (a sodium channel blocker) [Fig. 4(b)], suggesting presynaptic mechanisms. In addition, a small but substantial increase in the frequency of IPSCs also was observed in glut3+/− mice [Fig. 4(c)]. This suggests that increased seizure susceptibility cannot be explained by reduced inhibition, but rather by increased excitation.
Figure 4.
Whole-cell patch clamp recordings of spontaneous excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs) in cortical pyramidal neurons (frontal area, layers II-III) in male glut3+/− (HET) and male WT littermates at 12 months (a–c) are shown. At 12 months, an increase in the frequency of spontaneous glutamatergic synaptic events was observed (a), and this difference persisted in the presence of tetrodotoxin (TTX) (b), suggesting presynaptic influences. BIC, bicuculline. The frequency of spontaneous inhibitory postsynaptic currents also was increased (c). n = 17 cells in WT and n = 22 in glut3+/− mice. *P < 0.05, **P < 0.01 vs glut3+/− (HET).
Baseline aging investigations
In vivo brain MRS studies
The 8- to 10-month old glut3+/− male mice had lower hippocampal glucose content vs the WT counterpart (P < 0.05), whereas Lac, GABA, Glu, N-aspartic acid, and glutamine contents remained unchanged as measured in vivo by MRS (Table 2).
Table 2.
Brain Metabolite and Neurotransmitter Content by MRS
| WT (n = 10–14) | glut3+/− (n = 8–12) | |
|---|---|---|
| Glucose | 100 ± 14.2 | 60.2 ± 18.0a |
| Lac | 100 ± 11.0 | 81.4 ± 14.2 |
| GABA | 100 ± 10.6 | 82.9 ± 10.9 |
| Glu | 100 ± 8.3 | 83.8 ± 11.5 |
| N-aspartic acid | 100 ± 7.2 | 100.3 ± 11.2 |
| Glutamine | 100 ± 9.9 | 93.9 ± 9.2 |
Baseline hippocampal metabolite and neurotransmitter content in 8- to 10-month-old male glut3+/− mice depicted as percent of WT values (100%). MRS assessment of glucose, Lac, GABA, Glu, N-aspartic acid, and glutamine was compared between male WT and glut3+/− mice, where WT mice were calibrated at ∼100%.
P < 0.05 vs WT mice.
Brain β amyloid protein concentrations
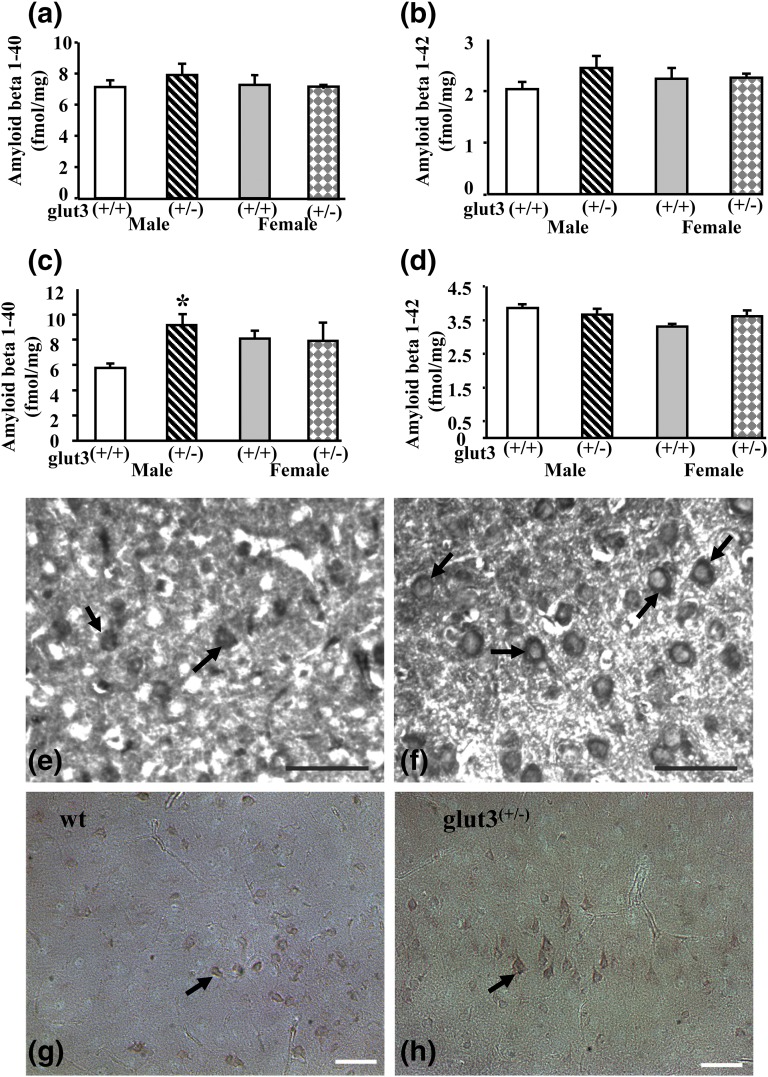
Amyloid β 1-40 and amyloid β 1-42 in male and female WT and glut3+/− mice were not different at 8 months of age [Fig. 5(a) and 5(b)]; however, at 24 months of age, sex-specific dichotomy was noted. Amyloid β 1-40 concentration was 1.4-fold higher in male but not female glut3+/− compared with WT mice (P < 0.05). Amyloid β 1-42 protein concentration, on the other hand, was not different between both the male and female WT and glut3+/− mice [Fig. 5(c) and 5(d)]. Representative cortical sections demonstrated a ∼2.6-fold increase (∼29 vs ∼11) in the number of neurons with β-amyloid (1-40 form) immunoreactivity [Fig. 5(e) and 5(f)] but with no change (∼23 vs ∼24) in the number of cells expressing neuronal β-1-42 immunoreactivity in male glut3+/− vs WT mice [Fig. 5(g) and 5(h)].
Figure 5.
Brain amyloid deposition. Brain β amyloid concentrations: amyloid β 1-40 and amyloid β 1-42 in male and female WT and glut3+/− mice at 8 months (a, b) and 24 months (c, d) of age. n = 6 to 11 in each group per age. *P < 0.05 vs male WT mice. Representative immunohistochemical staining of β-amyloid (1-40 form) protein (e, f) and β-amyloid 1-42 protein (g, h) in cerebral cortex of male WT (e, g) and glut3+/− mice (f, h). Positive immunohistochemical staining in neuronal cells is shown by arrows. Scale bars = 50 μm.
Ketogenic dietary interventional investigations
Neurobehavioral testing
Because the neuronal/synaptic investigations were performed in males alone, the neurobehavioral testing was also undertaken in male mice only.
Ultrasonic vocalization test.
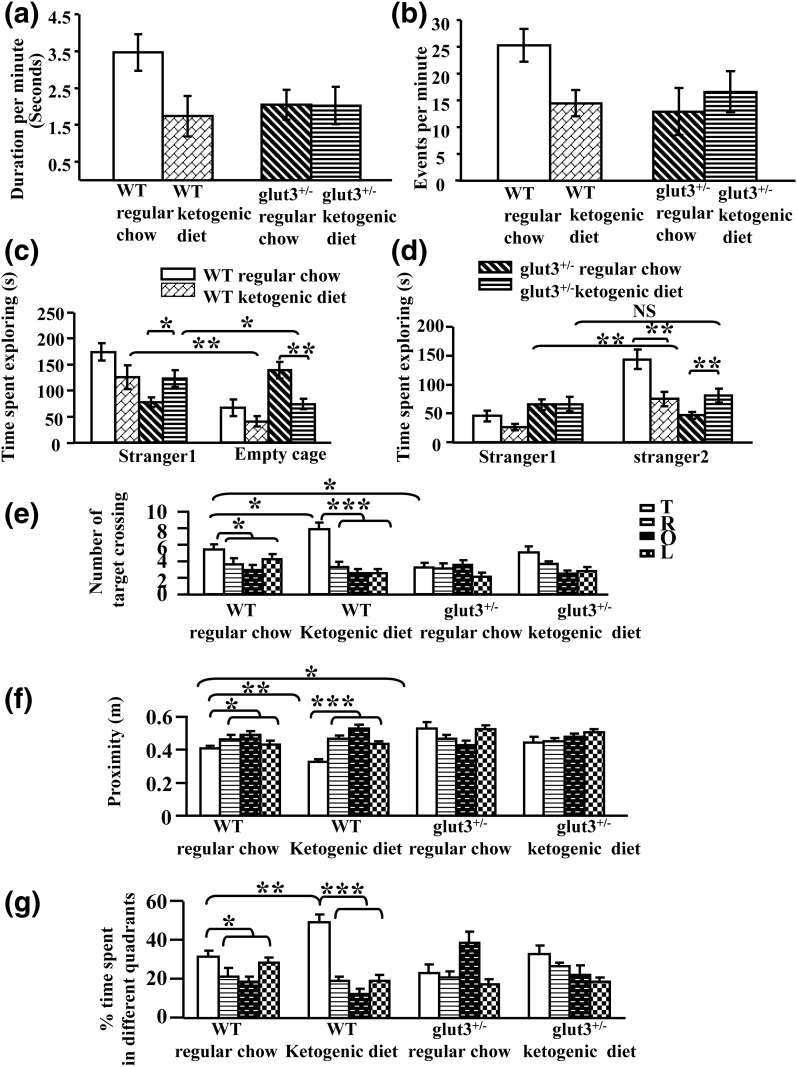
As previously described (8), Glut3 heterozygous null mice reduced the rates of vocalization under baseline conditions when mothers were maintained on a regular chow diet. The introduction of a ketogenic diet to the mothers during embryonic/fetal and neonatal development of the offspring demonstrated a trend toward decreased vocalizations in WT mice by reducing duration spent in vocalizations per minute (P = 0.15) [Fig. 6(a)] and the vocalization events per minute [Fig. 6(b)]. In contrast, ketogenic diet had no effect on the rates of vocalization in glut3+/− mice in either duration (P > 0.05) or in the number of events per minute (P > 0.05) [Fig. 6(a) and 6(b)].
Figure 6.
Neurobehavioral studies. Ultrasonic vocalization testing: ketogenic diet reduces vocalization as seen by decreased duration of vocalization per minute (0 to 6 minutes) (a) and vocalization events per minute (b) in WT mice but not in glut3+/− mice. WT male mice on regular chow (n = 38) or ketogenic diet (n = 7) and male glut3+/− mice on regular chow (n = 35) or ketogenic diet (n = 11). Sociability and social novelty: Glut3+/− genotype impaired social interaction (c). WT male mice on regular chow (n = 18) spent significantly more time exploring a conspecific than an empty cage in a social choice paradigm. WT-conspecific vs WT-empty cage: ***P < 0.001. Glut3+/− male mice on regular chow diet (n = 24), in contrast, spent more time exploring the empty cage than they spent interacting with the conspecific (**P < 0.004). Moreover glut3+/− mice spent significantly less time exploring the conspecific than WT controls (***P < 0.001), and therefore lacked normal levels of social exploration. A ketogenic diet significantly increased time spent interacting with the conspecific (*P < 0.05) and decreased the time exploring the empty cage (**P < 0.01), thus improving sociability in glut3+/− but not in WT male mice. In contrast, a ketogenic diet worsened the sociability in WT male mice (*P < 0.05 and **P < 0.01 respectively). n = 11 for WT on a ketogenic diet and n = 13 for glut3+/− mice on a ketogenic diet. Glut3+/− mice failed to show preference for the novel mouse in a social recognition task (d). WT: n = 18, ***P < 0.001; glut3+/−: n = 24, P = 0.106; time spent exploring unfamiliar mouse, WT vs glut3+/−: ***P < 0.001. A ketogenic diet significantly increased the time spent exploring the novel mouse, and therefore improved social novelty compared with familiar mouse in glut3+/− male mice (**P < 0.01). In contrast, a ketogenic diet significantly worsened social novelty in WT male mice (**P < 0.01). n = 11 for WT and n = 13 for glut3+/− mice both on a ketogenic diet. Morris water maze test examining spatial learning and memory: ketogenic diet increased spatial learning by enhancing number of target crossings (e), reducing proximity (f), and increasing time spent in the target quadrant (g) by WT male mice but not by glut3+/− male mice. WT mice on regular chow (n = 13) or ketogenic diet (n = 7) and glut3+/− mice on regular chow (n = 15) or ketogenic diet (n = 10), *P < 0.05 vs values in the target (T) quadrant in WT mice on regular chow diet. *P < 0.05, **P < 0.01, ***P < 0.001. L, left quadrant; O, opposite quadrant; R, right quadrant.
Sociability test.
WT mice at baseline when maintained on a regular chow diet spent more time actively exploring the cage containing the conspecific than the empty cage; this pattern reversed in glut3+/− mice that spent more time exploring the empty cage than the cage containing the stimulus mouse [Fig. 6(c)]. Moreover, glut3+/− mice spent significantly less time exploring the conspecific than WT controls [Fig. 6(d)] and therefore lacked normal levels of social exploration. Further, these glut3+/− mice, when subjected to a social recognition test, spent less time with the unfamiliar than the familiar mouse, supporting diminished aptitude for social novelty [Fig. 6(d)]. These results demonstrate that glut3+/− mice develop abnormal social interaction, including diminished social approach behavior and a lack of preference for social novelty. Other factors such as locomotor activity, which could influence the transition between chambers, and olfactory sensitivity, which could potentially affect sociability, were no different between the two mouse genotypes, as previously reported (8). Although the WT mice on a ketogenic diet unexpectedly demonstrated a worsening of sociability [Fig. 6(c)] and social novelty [Fig. 6(d)], the glut3+/− mice demonstrated an improvement in the 2 parameters when compared with the glut3+/− mice maintained on a chow diet (P = 0.011).
Morris water maze test.
Our previous investigations have shown that spatial cognition (learning and memory) was impaired in glut3+/− mice (8). To determine whether a ketogenic diet rescues the perturbed spatial cognition in glut3+/− mice, we compared spatial learning of both glut3+/− and WT mice maintained on regular chow and ketogenic diets using the hidden version of the Morris water maze. The WT mice crossed the exact target position more frequently than glut3+/− mice (P < 0.05) when maintained on a regular chow diet. In addition, WT mice demonstrated a closer proximity to the target (P < 0.05) and a greater percent time spent in target quadrant (P < 0.05) [Fig. 6(e–g)] than the glut3+/− counterpart. Introduction of a ketogenic diet improved spatial cognition in WT mice by demonstrating a higher number of target position crossing (P < 0.05), a closer proximity to the target (P < 0.05), and a greater percent time spent in target quadrant (P < 0.05). In contrast, the ketogenic diet failed to change the number of target crossings (P > 0.05), the proximity to the target (P > 0.05), and the percent of time spent in target quadrant (P > 0.05) in glut3+/− mice [Fig. 6(e–g)].
Continuous EEG monitoring
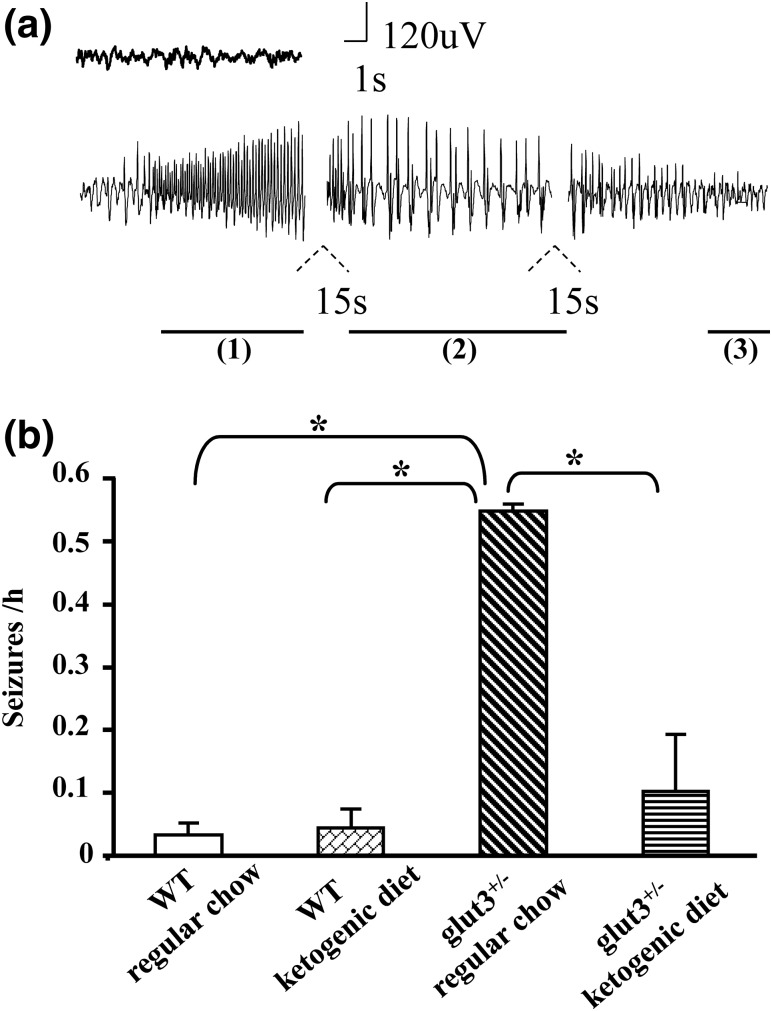
Continuous EEG telemetry recording at baseline demonstrated increased frequency of electrographic seizures in glut3+/− male mice compared with age-matched WT mice maintained on a regular chow diet, as previously described, at an earlier age (8) (P < 0.05) [Fig. 7(a) and 7(b)]. Ketogenic diet significantly decreased the average seizure frequency measured as episodes/hour in glut3+/− mice (P < 0.05), without any effect on the background average seizure frequency in WT mice (P > 0.05) [Fig. 7(b)].
Figure 7.
Continuous video-EEG monitoring. Epileptiform events in male WT and glut3+/− mice on regular chow and ketogenic diets. (a) Representative EEG tracing of a seizure event recorded, demonstrating tonic phase (1), clonic phase (2), and postictal depression (3). (b) Seizures per hour recorded in WT and glut3+/− mice maintained on regular chow and ketogenic diets, *P < 0.05 vs WT mice on a regular chow. WT mice on regular chow (n = 19) or ketogenic diet (n = 4) and glut3+/− mice on regular chow (n = 16) or ketogenic diet (n = 11).
Discussion
Although the phenotype of the glut3+/− heterozygous mice has been described previously by us and other groups (8, 9), here we have observed the neuro-metabolic phenotype to display age dependency and sex specificity through various phases of the life course.
Metabolic features
Male and not female glut3+/− adult mice expressed reduced CSF glucose with no perturbations in circulating glucose concentrations. This sex specificity exists despite a reduction in neuronal Glut3 in both males and females. However, a further ∼25% reduction in female neuronal Glut3 concentrations was evident vs the respective male WT and glut3+/− mice, reflecting reduced neural glucose demand. In addition, a reduction in CSF and circulating Lac concentrations but not ketones is evident only in the male glut3+/−, reflecting increased Lac utilization (8) by brain cellular elements and thereby depleting systemic availability. The reason behind these sex-specific differences may also stem from female mice exhibiting 2.5- to 3.5-fold higher circulating and CSF ketones than males, which may protect the neuronal fuel supply toward maintaining normal ATP in glut3+/− female mice. The metabolic features exhibited by the male glut3+/− mice reflect features of the human GLUT1 deficiency syndrome (G1DS) (19, 20) also reflected in glut1+/− mice (21), where no sex-specific differences were reported to date. Despite the metabolic changes seen in male glut3+/− mice, no differences were detected in baseline brain glucose transport or uptake between the genotypes or sexes, similar to our previous study (38) and those of others (6).
However, when we stressed the system by producing acute hypoglycemia, the male glut3+/− mice demonstrated a compensatory increase in brain glucose uptake, whereas females remained unchanged. The mechanism by which insulin-induced hypoglycemia was associated with enhanced brain glucose uptake in male glut3+/− mice alone remains unclear. Previously, insulin sensitivity of the brain was seen mainly in male adult rodents (22–24) and increased insulin receptors were evident only in the male postnatal brain (25–27) vs the age-matched female counterpart. However, glucose uptake by the whole brain is generally insulin-independent, thus stimulation of certain counterregulatory hormones in response to hypoglycemia may underlie the observed increase in brain glucose uptake (28–31). The glut3+/− females remaining unchanged perhaps was also related to the sufficient availability of ketones as an alternate fuel. Ultimately, no negative effect was realized on brain ATP concentrations from the compensatory changes and reliance on existing alternate fuels. However, our present results in male glut3+/− with insulin (which inhibits ketone production beyond baseline availability) are distinct from prior reports of no change in brain glucose uptake after starvation (over 48 hours)-induced hypoglycemia (which enhances production of ketones) in glut3+/− mice (6). Differences in our present study between glut3+/− males and females, and between our present insulin-induced hypoglycemia in glut3+/− males vs prior starvation-induced hypoglycemia in glut3+/− mice with no sex distinction, hint at alternate fuels, such as ketones, ameliorating some of the presenting features in male glut3+/− mice.
Because Glut3 deficiency is primarily limited to neurons, it is difficult to assess neuron-specific glucose transport in the midst of heterogeneity produced by various other cells of the intact brain. Indirect evidence, such as the diminution in CSF glucose concentrations despite normal plasma glucose concentrations in the male, attests to reduced neuronal transport. Redirection of enhanced glucose utilization by other nonneuronal cellular components of glut3+/− brains may be responsible for the lack of perturbed glucose uptake. Further, the concomitant reduction in CSF and plasma Lac may reflect enhanced neuronal Lac utilization as an alternate substrate while exposed to a high carbohydrate-containing regular chow diet. Although no change in hippocampal Lac content was observed with aging, previous studies revealed enhanced brain Lac uptake related to the adaptive increase in Mct2 concentrations in younger glut3+/− mice (8). Thus, the age-dependent compensatory role of increased brain Lac utilization may fuel and maintain the normal ATP concentrations in glut3+/− mice.
Neurotransmitters, synaptic activity, and molecular network
These metabolic changes were not associated with any perturbations in MRS-measured hippocampal neurotransmitter content. However, an increase in cortical glutamatergic activity was observed in 12-month-old glut3+/− male mice, when compensatory nutrient transporter adaptations (Glut1, Mct2) were no longer evident. Early on, at 2 months of age, when a compensatory increase in Glut1 and Mct2 were in play (8), we observed no differences in spontaneous synaptic activity. This suggests that lack of compensatory mechanisms, whether related to the availability of alternate fuels or transporter mechanisms, may be responsible for increased cortical excitability and seizure susceptibility in adult glut3+/− mice. A similar alteration is observed in a mouse model of Huntington disease that displays metabolic disturbances, diabetes, and heightened seizure susceptibility (16). In the present study, at 12 months of age, a substantial increase in spontaneous IPSCs was also observed in glut3+/− mice, perhaps reflecting increased excitatory synaptic activity on GABAergic cortical interneurons. Previous investigations demonstrated transient Glu excitation of cultured cerebellar granule neurons resulting in hyperpolarization of the mitochondrial membranes to instigate adenosine monophosphate–activated protein kinase–dependent translocation of Glut3 to the cell surface (32). Thus, Glut3 plays an important role in preserving neuronal energy metabolism and maintaining tolerance to Glu excitation.
In addition, various molecules responsible for presynaptic to postsynaptic interaction failed to change in glut3+/− mice, except for an increase in SAPAP1 and NLGN4 expression. Because our previous investigation revealed autism-like neurobehavior in younger glut3+/− mice (8), we tested the expression of certain synaptic markers (synaptophysin, MAP2) and genes that are disrupted in human autism (33, 34). We observed increased hippocampal NLGN4, whereas previously we had detected a decrease in NLGN3 at an earlier age (8). These changes, if translated into function, would enhance synaptic excitation. Spliced NLGNs interact with presynaptic neurexins, reinforcing dendritic-axonal contacts and thereby maintaining the excitatory (glutamatergic):inhibitory (GABAergic) synaptic neurotransmitter balance, an imbalance resulting in autism spectrum disorders (35). NLGN3 mediates inhibitory, whereas NLGN4 mediates excitatory synaptic neurotransmission; both of these mutations occur in human autism spectrum disorder (14, 36, 37). In addition, increased SAPAP1 expression (a scaffold protein that connects SHANK3 to PSD-95, ensuring integrity of postsynaptic dendrites) (38, 39) was seen in glut3+/− male mouse cortex. The increased SAPAP1 in glut3+/− male mice supports compensation against synaptic glutamatergic excitatory activity. These findings collectively suggest perturbed synaptic integrity, which underscores impaired cognition in the glut3 deficient mouse.
Amyloid accumulation
We then examined brain amyloid accumulation as a hallmark of neuronal compromise, particularly during aging. Glut3+/− mice at 6 to 8 months of age expressed EEG seizures with no changes in brain β-amyloid proteins in males and females. Further aging at 24 months revealed an increase in Aβ 1-40 protein only in male glut3+/− vs WT mice, with no change in Aβ 1-42 protein. Amyloid protein accumulation causes neuronal destruction, as seen in human Alzheimer’s disease. Although the 1-42 form is noted to be the fibrillary form responsible for neuronal loss, the 1-40 form, not culminating in neuronal loss, is nevertheless toxic, resulting in an inflammatory response leading to learning and memory loss. Thus, Aβ 1-40 protein is responsible for early signs of Alzheimer’s disease (40–42). This neuronal accumulation of Aβ 1-40 may subsequently occur after an excitotoxic insult that results from enhanced Glu excitatory activity seen in glut3+/− mice.
Ketogenic dietary intervention, seizures, and neurobehaviors
G1DS patients are currently treated with a ketogenic diet for controlling seizures in infancy and preventing developmental delays (43, 44). Adult G1DS patients adapt and overcome their disabilities when placed in time on a ketogenic diet during infancy and childhood, before the establishment of neurologic injury (19, 20). Unlike G1DS, in which gait and motor function are also affected, Glut3-deficient mice display disruption in vocalization, stereotypies, and deficiency in social interaction, spatial learning, and memory. These murine phenotypic observations reflect neurodevelopmental delays reported in infants with glut3 gene copy number variations (45, 46). A dietary intervention would prove highly useful. The ketogenic dietary intervention in our present study was dramatic in ameliorating EEG seizures and improving sociability, but failed to affect perturbed vocalizations, spatial learning, and memory, which are characteristic of glut3+/− mice. These results support that ketones in the face of neuroglucopenia and enhanced Lac utilization help fuel neuronal energy requirement, improving only certain clinical features. This also explains the neuroprotection seen at baseline in glut3+/− female mice resulting from higher ketone availability.
In contrast, ketogenic diet in WT male mice had a detrimental effect on vocalizations and sociability, while demonstrating enhanced to normal spatial learning and memory. Thus, it appears that ketones in the presence of adequate glucose availability on a normal genotypic background worsen social and communication skills without a negative effect on cognition. These findings support the premise that, under normal conditions, ketones are not the preferred fuel for neuronal energy.
Conclusions
Our investigations in glut3+/− mice rests on (1) age-dependency of neurologic perturbations related to the presence or absence of compensatory mechanisms involving availability of alternate fuels and other brain glucose and monocarboxylate transporter isoforms; (2) sex-specificity seen as neuroprotection of females resulting from higher circulating and CSF ketone concentrations and/or lower brain Glut3 concentrations; (3) synaptic perturbations suggestive of an imbalance between glutamatergic:GABAergic synaptic molecules, resulting in age-related glutamatergic excitotoxicity; (4) culmination in neuronal β-amyloid 1-40 accumulation with aging only in males; and (5) introduction of a ketogenic diet early in life ameliorating EEG seizures and improving sociability, while not affecting vocalization and cognitive deficits in males. We conclude that the glut3+/− genotype with associated metabolic perturbations underlie the ultimate male disadvantaged neurobehavioral phenotype. Given that developmental delays have been associated with glut3 gene variations, we speculate that timely ketogenic intervention in human disorders that carry copy number variations of the glut3 gene may prevent certain but not all features of such neurodevelopmental disorders (45, 47).
Acknowledgments
We acknowledge the assistance of Don Shin with the electroencephalogram studies and Lynn Talton in the University of California Los Angeles (University of California, Los Angeles) Behavioral Testing Core Laboratory. We thank Alcino Silva (University of California, Los Angeles) for his initial help in setting up the behavioral studies and Amit Ganguly for assisting with maintenance and supply of genotyped mice for some of the studies.
Acknowledgments
This work was supported by the National Institutes of Child Health and Human Development Grants 33997 and 81206 (to S.U.D.) and 4612 (to M.S.L.). The Cell, Circuits and Systems Analysis Core was supported by National Institutes of Health Grant U54HD087101 (to M.S.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATP
- adenosine triphosphate
- CSF
- cerebrospinal fluid
- EEG
- electroencephalogram
- EPSC
- excitatory postsynaptic current
- GABA
- γ-aminobutyric acid
- Glu
- glutamate
- IPSC
- inhibitory postsynaptic current
- Lac
- lactate
- mRNA
- messenger RNA
- MRS
- magnetic resonance spectroscopy
- PET
- positron emission tomography
- WT
- wild-type
- β-HB
- β-hydroxybutyrate.
References
- 1.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27(11):1766–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devaskar S, Zahm DS, Holtzclaw L, Chundu K, Wadzinski BE. Developmental regulation of the distribution of rat brain insulin-insensitive (Glut 1) glucose transporter. Endocrinology. 1991;129(3):1530–1540. [DOI] [PubMed] [Google Scholar]
- 3.Mantych GJ, James DE, Chung HD, Devaskar SU. Cellular localization and characterization of Glut 3 glucose transporter isoform in human brain. Endocrinology. 1992;131(3):1270–1278. [DOI] [PubMed] [Google Scholar]
- 4.Sankar R, Thamotharan S, Shin D, Moley KH, Devaskar SU. Insulin-responsive glucose transporters-GLUT8 and GLUT4 are expressed in the developing mammalian brain. Brain Res Mol Brain Res. 2002;107(2):157–165. [DOI] [PubMed] [Google Scholar]
- 5.Shin BC, McKnight RA, Devaskar SU. Glucose transporter GLUT8 translocation in neurons is not insulin responsive. J Neurosci Res. 2004;75(6):835–844. [DOI] [PubMed] [Google Scholar]
- 6.Stuart CA, Ross IR, Howell ME, McCurry MP, Wood TG, Ceci JD, Kennel SJ, Wall J. Brain glucose transporter (Glut3) haploinsufficiency does not impair mouse brain glucose uptake. Brain Res. 2011;1384:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganguly A, McKnight RA, Raychaudhuri S, Shin BC, Ma Z, Moley K, Devaskar SU. Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am J Physiol Endocrinol Metab. 2007;292(5):E1241–E1255. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Fung C, Shin D, Shin BC, Thamotharan S, Sankar R, Ehninger D, Silva A, Devaskar SU. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol Psychiatry. 2010;15(3):286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung C, Evans E, Shin D, Shin BC, Zhao Y, Sankar R, Chaudhuri G, Devaskar SU. Hypoxic-ischemic brain injury exacerbates neuronal apoptosis and precipitates spontaneous seizures in glucose transporter isoform 3 heterozygous null mice. J Neurosci Res. 2010;88(15):3386–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taschereau RV, Chatziioannou NT. Calibration and data standardization of a prototype bench-top pre-clinical CT. In: A.F. Institute of Electrical and Electronics Engineers Nuclear Science Symposium & Medical Imaging Conference; November 8–15, 2014; Seattle, WA. [Google Scholar]
- 11.Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2(3):131–137. [DOI] [PubMed] [Google Scholar]
- 12.Zovein A, Flowers-Ziegler J, Thamotharan S, Shin D, Sankar R, Nguyen K, Gambhir S, Devaskar SU. Postnatal hypoxic-ischemic brain injury alters mechanisms mediating neuronal glucose transport. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R273–R282. [DOI] [PubMed] [Google Scholar]
- 13.Ganguly A, Devaskar SU. Glucose transporter isoform-3-null heterozygous mutation causes sexually dimorphic adiposity with insulin resistance. Am J Physiol Endocrinol Metab. 2008;294(6):E1144–E1151. [DOI] [PubMed] [Google Scholar]
- 14.State MW. The genetics of child psychiatric disorders: focus on autism and Tourette syndrome. Neuron. 2010;68(2):254–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin BC, Dai Y, Thamotharan M, Gibson LC, Devaskar SU. Pre- and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J Neurosci Res. 2012;90(6):1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings DM, André VM, Uzgil BO, Gee SM, Fisher YE, Cepeda C, Levine MS. Alterations in cortical excitation and inhibition in genetic mouse models of Huntington’s disease. J Neurosci. 2009;29(33):10371–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomi M, Zhao Y, Thamotharan S, Shin BC, Devaskar SU. Early life nutrient restriction impairs blood-brain metabolic profile and neurobehavior predisposing to Alzheimer’s disease with aging. Brain Res. 2013;1495:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin BC, Suzuki M, Inukai K, Anai M, Asano T, Takata K. Multiple isoforms of the regulatory subunit for phosphatidylinositol 3-kinase (PI3-kinase) are expressed in neurons in the rat brain. Biochem Biophys Res Commun. 1998;246(2):313–319. [DOI] [PubMed] [Google Scholar]
- 19.Klepper J, Leiendecker B. GLUT1 deficiency syndrome--2007 update. Dev Med Child Neurol. 2007;49(9):707–716. [DOI] [PubMed] [Google Scholar]
- 20.De Vivo DC, Leary L, Wang D. Glucose transporter 1 deficiency syndrome and other glycolytic defects. J Child Neurol 2002;17(Suppl 3):3S15–3S25. [PubMed] [Google Scholar]
- 21.Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15(7):1169–1179. [DOI] [PubMed] [Google Scholar]
- 22.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93(4):1339–1344. [DOI] [PubMed] [Google Scholar]
- 23.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin [published correction appears in Diabetes 2007;56(10):2649]. Diabetes. 2006;55(4):978–987. [DOI] [PubMed] [Google Scholar]
- 24.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52(3):682–687. [DOI] [PubMed] [Google Scholar]
- 25.Hami J, Sadr-Nabavi A, Sankian M, Haghir H. Sex differences and left-right asymmetries in expression of insulin and insulin-like growth factor-1 receptors in developing rat hippocampus. Brain Struct Funct. 2012;217(2):293–302. [DOI] [PubMed] [Google Scholar]
- 26.Haghir H, Rezaee AA, Nomani H, Sankian M, Kheradmand H, Hami J. Sexual dimorphism in expression of insulin and insulin-like growth factor-I receptors in developing rat cerebellum. Cell Mol Neurobiol. 2013;33(3):369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu WH, Huber R, Riepe MW. Gender- and region-specific expression of insulin receptor protein in mouse brain: effect of mild inhibition of oxidative phosphorylation. J Neural Transm (Vienna). 2007;114(3):373–377. [DOI] [PubMed] [Google Scholar]
- 28.Vavaiya KV, Paranjape SA, Briski KP. Testicular regulation of neuronal glucose and monocarboxylate transporter gene expression profiles in CNS metabolic sensing sites during acute and recurrent insulin-induced hypoglycemia. J Mol Neurosci. 2007;31(1):37–46. [DOI] [PubMed] [Google Scholar]
- 29.Molina PE, Abumrad NN. Contribution of excitatory amino acids to hypoglycemic counter-regulation. Brain Res. 2001;899(1-2):201–208. [DOI] [PubMed] [Google Scholar]
- 30.Cranston I, Reed LJ, Marsden PK, Amiel SA. Changes in regional brain (18)F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counter-regulatory failure. Diabetes. 2001;50(10):2329–2336. [DOI] [PubMed] [Google Scholar]
- 31.Boyle PJ. Alteration in brain glucose metabolism induced by hypoglycaemia in man. Diabetologia. 1997;40(Suppl 2):S69–S74. [DOI] [PubMed] [Google Scholar]
- 32.Weisová P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29(9):2997–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17(4):434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubenstein JL. Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Curr Opin Neurol. 2010;23(2):118–123. [DOI] [PubMed] [Google Scholar]
- 36.Bottos A, Rissone A, Bussolino F, Arese M. Neurexins and neuroligins: synapses look out of the nervous system. Cell Mol Life Sci. 2011;68(16):2655–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krueger DD, Tuffy LP, Papadopoulos T, Brose N. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr Opin Neurobiol. 2012;22(3):412–422. [DOI] [PubMed] [Google Scholar]
- 38.Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schütt J, Falley K, Richter D, Kreienkamp HJ, Kindler S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J Biol Chem. 2009;284(38):25479–25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda S, Sato N, Niisato K, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Kano M, Morishita R. Validation of abeta1-40 administration into mouse cerebroventricles as an animal model for Alzheimer disease. Brain Res. 2009;1280:137–147. [DOI] [PubMed] [Google Scholar]
- 41.Piermartiri TC, Figueiredo CP, Rial D, Duarte FS, Bezerra SC, Mancini G, de Bem AF, Prediger RD, Tasca CI. Atorvastatin prevents hippocampal cell death, neuroinflammation and oxidative stress following amyloid-β(1-40) administration in mice: evidence for dissociation between cognitive deficits and neuronal damage. Exp Neurol. 2010;226(2):274–284. [DOI] [PubMed] [Google Scholar]
- 42.Prediger RD, Medeiros R, Pandolfo P, Duarte FS, Passos GF, Pesquero JB, Campos MM, Calixto JB, Takahashi RN. Genetic deletion or antagonism of kinin B(1) and B(2) receptors improves cognitive deficits in a mouse model of Alzheimer’s disease. Neuroscience. 2008;151(3):631–643. [DOI] [PubMed] [Google Scholar]
- 43.Coman DJ, Sinclair KG, Burke CJ, Appleton DB, Pelekanos JT, O’Neil CM, Wallace GB, Bowling FG, Wang D, De Vivo DC, McGill JJ. Seizures, ataxia, developmental delay and the general paediatrician: glucose transporter 1 deficiency syndrome. J Paediatr Child Health. 2006;42(5):263–267. [DOI] [PubMed] [Google Scholar]
- 44.Verrotti A, D’Egidio C, Agostinelli S, Gobbi G. Glut1 deficiency: when to suspect and how to diagnose? Eur J Paediatr Neurol. 2012;16(1):3–9. [DOI] [PubMed] [Google Scholar]
- 45.Roeske D, Ludwig KU, Neuhoff N, Becker J, Bartling J, Bruder J, Brockschmidt FF, Warnke A, Remschmidt H, Hoffmann P, Müller-Myhsok B, Nöthen MM, Schulte-Körne G. First genome-wide association scan on neurophysiological endophenotypes points to trans-regulation effects on SLC2A3 in dyslexic children. Mol Psychiatry. 2011;16(1):97–107. [DOI] [PubMed] [Google Scholar]
- 46.Mlynarski EE, Sheridan MB, Xie M, Guo T, Racedo SE, McDonald-McGinn DM, Gai X, Chow EW, Vorstman J, Swillen A, Devriendt K, Breckpot J, Digilio MC, Marino B, Dallapiccola B, Philip N, Simon TJ, Roberts AE, Piotrowicz M, Bearden CE, Eliez S, Gothelf D, Coleman K, Kates WR, Devoto M, Zackai E, Heine-Suñer D, Shaikh TH, Bassett AS, Goldmuntz E, Morrow BE, Emanuel BS; International Chromosome 22q11.2 Consortium . Copy-number variation of the glucose transporter gene SLC2A3 and congenital heart defects in the 22q11.2 deletion syndrome. Am J Hum Genet. 2015;96(5):753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lesch KP, Selch S, Renner TJ, Jacob C, Nguyen TT, Hahn T, Romanos M, Walitza S, Shoichet S, Dempfle A, Heine M, Boreatti-Hümmer A, Romanos J, Gross-Lesch S, Zerlaut H, Wultsch T, Heinzel S, Fassnacht M, Fallgatter A, Allolio B, Schäfer H, Warnke A, Reif A, Ropers HH, Ullmann R. Genome-wide copy number variation analysis in attention-deficit/hyperactivity disorder: association with neuropeptide Y gene dosage in an extended pedigree. Mol Psychiatry. 2011;16(5):491–503. [DOI] [PubMed] [Google Scholar]