Abstract
The broadly expressed transcriptional coregulator LDB1 is essential for β-cell development and glucose homeostasis. However, it is unclear whether LDB1 has metabolic roles beyond the β-cell, especially under metabolic stress. Global Ldb1 deletion results in early embryonic lethality; thus, we used global heterozygous Ldb1+/− and inducible β-cell–specific Ldb1-deficient (Ldb1Δβ-cell) mice. We assessed glucose and insulin tolerance, body composition, feeding, and energy expenditure during high-fat diet exposure. Brown adipose tissue (BAT) biology was evaluated by thermogenic gene expression and LDB1 chromatin immunoprecipitation analysis. We found that partial loss of Ldb1 does not impair the maintenance of glucose homeostasis; rather, we observed improved insulin sensitivity in these mice. Partial loss of Ldb1 also uncovered defects in energy expenditure in lean and diet-induced obese (DIO) mice. This decreased energy expenditure during DIO was associated with significantly altered BAT gene expression, specifically Cidea, Elovl3, Cox7a1, and Dio2. Remarkably, the observed changes in energy balance during DIO were absent in Ldb1Δβ-cell mice, despite a similar reduction in plasma insulin, suggesting a role for LDB1 in BAT. Indeed, LDB1 is expressed in brown adipocytes and occupies a regulatory domain of Elovl3, a gene crucial to normal BAT function. We conclude that LDB1 regulates energy homeostasis, in part through transcriptional modulation of critical regulators in BAT function.
We report that the Ldb1 transcriptional coregulator has roles in energy homeostasis, in part through transcriptional modulation of critical regulators of brown adipose tissue function.
Obesity, imparted by reduced physical activity and a concomitant increase in high-fat food intake, has become a worldwide epidemic with life-altering comorbidities including type 2 diabetes and cardiovascular disease (1, 2). Of the strategies to combat weight gain and metabolic syndrome in humans, many seek to exploit the metabolic benefits of thermogenic brown adipocyte depots. As a result, elucidating the pathways that promote the development and activation of brown adipose tissue (BAT) is of particular interest.
Recently, efforts have revealed transcription factors and interacting coregulators that impart brown adipose cell identity and function (3, 4). In particular, the adipogenic master regulator peroxisome proliferator-activated receptor-γ (PPARγ) is required for the development of both the white and brown adipose lineages (5, 6), albeit insufficient to solely drive the BAT program. Notably, PPARγ positively regulates uncoupling protein 1 (Ucp1), a gene required for mitochondrial proton leak and heat generation in brown adipocytes (7). This regulation is facilitated by the zinc-finger methyltransferase PR domain-containing protein 16, a BAT-enriched coregulator that is necessary and sufficient in driving BAT-selective transcriptional programs (8). Of note, this occurs in part through physical interactions with PPARγ that regulate core BAT genes, including Ucp1, Diodinase type 2 (Dio2), and the cold-induced coregulator PPARγ-coactivator-1α. With increased focus on BAT biology, unique regulators required for brown adipose differentiation programs or energy expenditure (EE) and BAT-related thermogenesis are being uncovered.
We have been interested in the LIM (Lin11, Isl1, Mec3)-domain binding coregulator LDB1, especially in the context of pancreatic islet β-cell development and function (9). LDB1 is a scaffolding factor that avidly binds LIM-domains of the LIM-homeodomain (LIM-HD), and LIM-Only (LMO) classes of transcriptional regulators, which are broadly expressed throughout embryogenesis and in adult tissues. Additionally, LDB1-LMO complexes interact with basic helix-loop-helix and Gata classes of transcription factors, for example during erythropoiesis (10). Thus, our overarching hypothesis is that LDB1 integrates the function of LIM-domain transcription factor complexes throughout development and in the maintenance of adult tissue function.
Based on the importance of LDB1 in pancreatic development and function(9, 11), we initially set out to investigate the role of LDB1 in adult glucose metabolism, especially with regard to pancreatic islet responses to diet-induced obesity (DIO). Hence, we used a global Ldb1 heterozygote (Ldb1+/−) model challenged with a high-fat diet (HFD). These mice had an expectedly mild pancreatic phenotype, especially when compared with islet-specific constitutive or adult-inducible Ldb1 deficiency models (9, 11). Most notable was a reduction in plasma insulin levels that was likely a secondary response to increased insulin sensitivity. Surprisingly, the DIO Ldb1+/− mice had reduced food intake coupled with normal body weight gain on HFD. Further, Ldb1+/− mice had reduced EE regardless of diet, which was associated with impaired expression of thermogenic genes in the BAT of Ldb1+/− mice. BAT-expressed LDB1 may contribute to these effects, as LDB1 is expressed in BAT and binds a key promoter domain of Elovl3, encoding an elongation enzyme required for the synthesis of very long-chain fatty acids in brown adipocytes, thus facilitating early BAT recruitment and function during cold stress (12). Strikingly, β-cell–specific LDB1 deficiency lacks this BAT phenotype, although plasma insulin is similarly reduced. Taken together, we found that LDB1 is required for normal EE and appears to be a unique regulator of BAT function.
Materials and Methods
Mouse models
Ldb1 null allele mice (Ldb1-) were described (13). Male Ldb1+/− and control Ldb1+/+ littermates (indicated as CTL) were maintained on standard chow (4.7 kcal%, #7917, Envigo, Indianapolis, IN) until 13 weeks of age and then given ad libitum access to HFD for 8 weeks (58.0 kcal% fat, #D12331 Research Diets, New Brunswick, NJ). The conditional, inducible β-cell LDB1 knockout (Ldb1Δβ-cell) was generated by crossing floxed Ldb1 (Ldb1F/F) (14) and transgenic MIP-CreERT (15) mice. Cre activity was induced via dosing tamoxifen (T5648; Sigma Aldrich, St. Louis, MO) at 150 μg/g body weight in 8- to 10-week male Ldb1Δβ-cell mice and control Ldb1F/F littermates (indicated as CTL). Tamoxifen was dissolved in corn oil and administered by gavage to all mice on 5 consecutive days followed by a 2-day rest, and then repeated for another five daily doses [as described Ediger et al.in (11)]. Mice were allowed a 2-week washout period prior to initiation of HF-feeding. Ldb1Δβ-cell and control littermates were maintained on standard chow until 13 weeks of age when they were provided ad libitum access to HFD for 4 weeks. All mouse lines were maintained on a mixed (C57BL/6) background. Mice were single or group housed on a 12-hour:12-hour light:dark cycle (light on from 0600 to 1800 hours) at 25°C ± 1°C and constant humidity with free access to food and water except as noted. All studies were approved by and performed according to the guidelines of the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Body composition and indirect calorimetry
Weekly food intake and body weight measurements were collected. Body composition was measured immediately before and following 8-week (Ldb1+/− and controls) or 4-week (Ldb1Δβ-cell and controls) HF feeding using noninvasive nuclear magnetic resonance spectroscopy (EchoMRI; Echo Medical Systems) at the University of Alabama at Birmingham Nutrition Obesity Research Center Small Animal Phenotyping Core. EE and home cage activity were assessed using a combined indirect calorimetry system (Comprehensive Laboratory Animal Monitoring System; Columbus Instruments) as previously described (16). Volume of O2 consumption and CO2 production (VO2 and VCO2, respectively) were measured every 15 minutes to determine respiratory quotient (RQ) and EE. Home cage locomotor activity was determined using a multidimensional infrared light beam system.
Glucose and insulin tolerance tests
Intraperitoneal (IP) glucose and insulin tolerance tests were performed by injection of glucose (1.5 [HFD] or 2.0 [standard chow] g/kg, 20% w/v d-glucose [Sigma] in 0.9% w/v saline) or regular human insulin (0.75 U/kg [Lilly Humalog] in saline) to 5-hour fasted mice. Blood glucose was measured with a TheraSense Freestyle Glucometer immediately before and 15, 30, 45, 60, and 120 minutes after injection.
Tissue isolation
Ad libitum-fed control and Ldb1+/− mice were sedated with pentobarbital and decapitated. Plasma was collected from trunk blood, and tissues were harvested and immediately stored at –80°C until analysis.
Immunofluorescence, immunohistochemistry, and immunoblot analysis
Pancreata were prepared for immunofluorescence staining as described (9). Primary antibodies included goat α-LDB1 (1:1000 Santa Cruz) and guinea pig α-Insulin (1:1000 Dako) (Table 1). Cy-2 and Cy-3 conjugated α-goat and α-guinea pig secondary antibodies (1:500; Jackson ImmunoResearch) were used for detection. Images were generated using an Olympus IX81 and processed by CellSens Dimensions (Olympus) or ImageJ (National Institutes of Health) software. Western blotting was performed as described (9). Interscapular brown adipose depots from C57BL/6 mice were dissected, fixed in 4% paraformaldehyde for 24 hours at 4°C, and embedded in paraffin. Sections were rehydrated, and antigen retrieval was performed in 0.01M sodium citrate buffer using a pressure cooker. Sections were blocked with CAS-Block (Invitrogen), primary antibodies (goat α-Ldb1, 1:250; rabbit α-PPARγ, 1:500 Santa Cruz; mouse α-ISL1, 1:1000 DSHB) were added overnight (4°C), and α-goat and α-rabbit secondary antibodies were applied for 40 minutes (37°C). Vectastain Elite ABC Kit (Vector) with Peroxidase Substrate Kit 3,3′-diaminobenzidine (Vector) was used for signal detection, and nuclei were counterstained with hematoxylin.
Table 1.
Antibodies Used in This Study
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|
| LDB1 | CLIM-2 | Santa Cruz | Goat; polyclonal | 1:250–1:1000 |
| β-Actin | Cell Signaling | Rabbit; monoclonal | 1:1000 | |
| PPARγ | Santa Cruz | Rabbit; polyclonal | 1:500 | |
| Islet-1 | 39.4D5 | DSHB | Mouse; monoclonal | 1:1000 |
| Elovl3 | Santa Cruz | Goat; polyclonal | 1:1000 | |
| Insulin | Dako | Guinea pig; polyclonal | 1:1000 |
Quantitative real-time PCR
RNA was isolated using the RNeasy Lipid Mini Kit (Qiagen) according to the manufacturer’s instructions. Complementary DNA was synthesized by reverse transcription polymerase chain reaction (PCR) using SuperScript III, DNase treatment, and anti-RNase treatment (Invitrogen). Single-gene quantitative PCR was performed as previously described (16). Data were normalized to housekeeping genes Gapdh (islet) and Hprt or 36B4 (BAT). See Supplemental Table 1 (61.8KB, pdf) for list of probes and primer sets.
ChIP
Chromatin immunoprecipitation (ChIP) was performed as described (9, 17). Briefly, brown adipose lobes were dissected from C57Bl/6 wild-type mice, phosphate-buffered saline washed, minced, fixed with 1% formaldehyde, and glycine quenched. Protein-DNA fragments were prepared by sonication (Bioruptor XL; Diagenode), precleared, and then incubated overnight at 4°C with α-LDB1 (Santa Cruz) or control goat immunoglobulin G (IgG). Chromatin complexes were precipitated with Protein G Dynabeads (Life Technologies) at 4°C for 4 hours, washed, and eluted, and crosslinks were reversed. Quantitative PCR was performed on the IP DNA using SYBR Green PCR master mix (Bio-Rad) and a LightCycler 480 II (Roche). Enriched ChIP DNA was normalized to Albumin promoter DNA enrichment (18) and calculated relative to IgG, set as onefold (ΔΔCt). ChIP experiments were performed four times with independent chromatin preparations.
Plasma hormone analyses
Plasma insulin was quantified via enzyme-linked immunosorbent assay assay according to the manufacturer’s instructions (Crystal Chem). Plasma analyte levels were determined using Bio-Plex Pro Mouse Diabetes 8-Plex Assay (Bio-Rad Laboratories). All other analytes were measured from terminal plasma collection per the manufacturer’s instructions. Analytical variation of assays was assumed as reported by the manufacturer.
Statistics
All data are represented as mean ± standard error of the mean (SEM). Glucose disappearance rate (kg) was defined as Δ blood glucose/min. Statistical significance was determined using unpaired Student t tests or, where appropriate, one- and two-way analysis of variance with multiple-comparison Tukey and Sidak post-tests, respectively, which was completed using GraphPad Prism version 6.0 (GraphPad Software). EE data were analyzed via analysis of covariants (SAS v9.4) as recommended by Tschöp et al. (19) and Friedman’s nonparametric 2-way analysis of variance for repeated measures. Statistical significance was assigned when P < 0.05.
Results
LDB1 heterozygosity does not impair glucose homeostasis under high-fat feeding
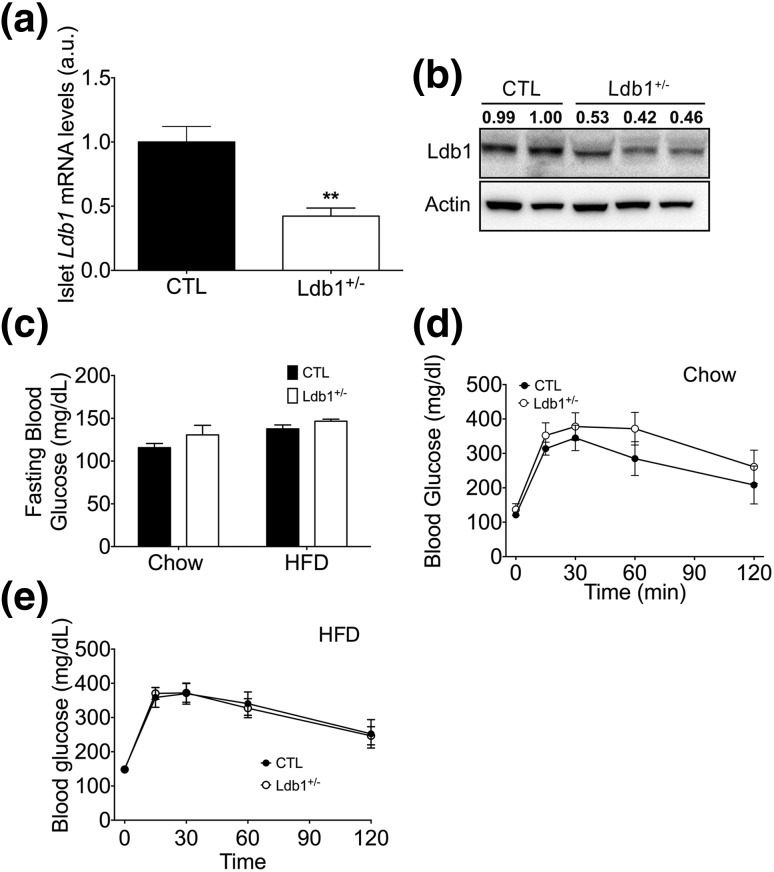
Because LDB1 is required for β-cell development and subsequent postnatal function (9, 11) and Ldb1 null mice (i.e., Ldb1−/−) are uninformative due to early embryonic lethality (13), we assessed glucose homeostasis in a phenotypically mild global Ldb1 heterozygous mouse (Ldb1+/− )(13) challenged by chronic HF feeding. To corroborate that LDB1 heterozygosity translated to reduced protein and messenger RNA (mRNA) levels, we quantified pancreatic Ldb1 expression and confirmed a 50% reduction at the transcript and protein levels in Ldb1+/− mice [Fig. 1(a) and 1(b)]. However, despite reduced LDB1, we found that low- or high-fat diet does not significantly affect fasting glycemia in Ldb1+/− and control littermates. Furthermore, when challenged with IP glucose, glucose tolerance in Ldb1+/− and controls was not statistically different on chow diet or following 10-week HFD [Fig. 1(c–e)]. These results suggest that maintaining one Ldb1 allele is sufficient for glucose homeostasis in healthy and DIO animals.
Figure 1.
Regulation of glucose metabolism and insulin action in Ldb1+/− and control (CTL) littermates. (a, b) Islet LDB1 mRNA and protein levels following 14-week HF feeding. mRNA levels were normalized to Gapdh. Western blotting of nuclear extracts isolated from total pancreas. LDB1 band densitometry is included above each lane, relative to Actin loading control. (c) Fasting blood glucose on chow and following 10 weeks of HFD. Blood glucose excursion following an IP bolus of glucose (1.5 g/kg) on (d) chow diet and (e) after 8 weeks of HFD. Data represented as mean ± SEM. **P < 0.01; n = 10 to 14 mice/group.
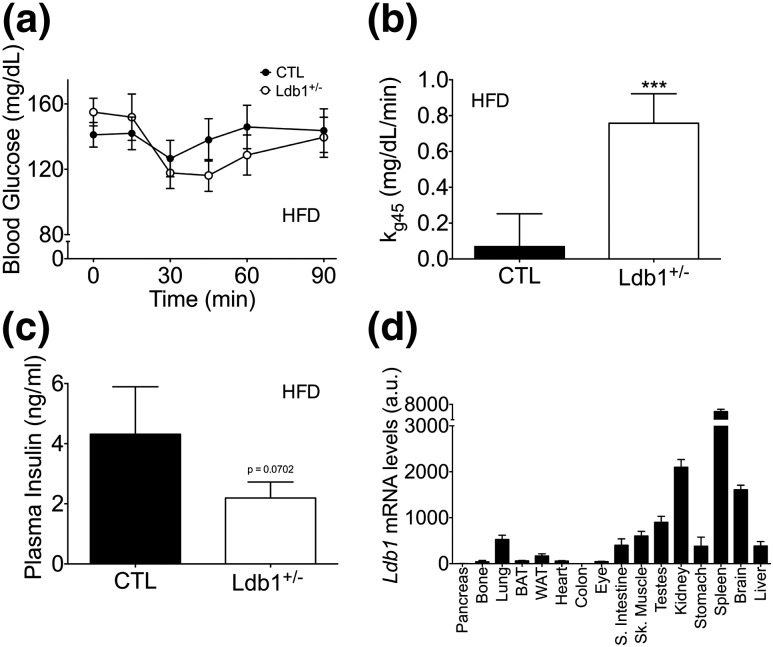
We next assessed the impact of Ldb1 on insulin action by challenging HF-fed Ldb1+/− and control littermates with an IP insulin bolus. Ldb1+/− mice displayed a subtle enhancement in insulin action as indicated by increased blood-glucose disappearance rate (kg) from 0 to 45 minutes [Fig. 2(a) and 1(b)]. Enhanced insulin sensitivity is further supported by the trend toward reduced ad libitum circulating insulin levels [P = 0.07; Fig. 2(c)]. We also observed a mild, yet significant decrease in islet mRNA levels of Insulin 2 (Ins2) and Slc2a2 (encoding the glucose transporter Glut2) in the Ldb1+/− mice following 14 weeks of HF feeding (Supplemental Fig. 1 (33.4MB, tiff) ). This is consistent with our previous findings in pancreas-specific LDB1 deficiency models (9, 11), but could also be secondary to an appropriate adaptive response to the observed improvement in insulin sensitivity. Collectively, these data confirm our prior findings (9) and indicate that Ldb1 mediates systemic insulin action but is haplosufficient for the maintenance of glucose homeostasis.
Figure 2.
Ldb1+/− mice have enhanced insulin action during HF feeding compared with control (CTL) littermates. (a) Blood glucose excursion following an IP bolus of insulin (0.75 U/kg) after 8 weeks of HFD. (b) kg between time point 0 (time of injection) and time point 45 minutes (nadir of blood glucose curve) during the insulin tolerance test. (c) Ad libitum plasma insulin levels after 10 weeks on HFD. Data represented as mean ± SEM. ***P < 0.001; n = 10 to 14 mice/group. (d) Relative Ldb1 expression across various tissues in 8-week-old wild-type C57BL/6 male mice (n = 3). mRNA levels were normalized to Gapdh, and the lowest expressing tissue (colon) was set to onefold. a.u., arbitrary units.
LDB1 is broadly expressed across metabolic tissues
To assess potential metabolic roles for LDB1 beyond the pancreas, we first performed an mRNA expression screen across various tissues. Ldb1 mRNA was found in many tissues required for metabolic action, including white and brown adipose, liver, skeletal muscle, and brain [Fig. 2(d)]. The low level of Ldb1 mRNA in the total pancreas sample is likely due to reduced expression in acinar cells (9) and not low template quality, as Insulin I was robustly expressed in these samples (Supplemental Fig. 2 (33.8MB, tiff) ).
LDB1 regulates energy balance in DIO mice
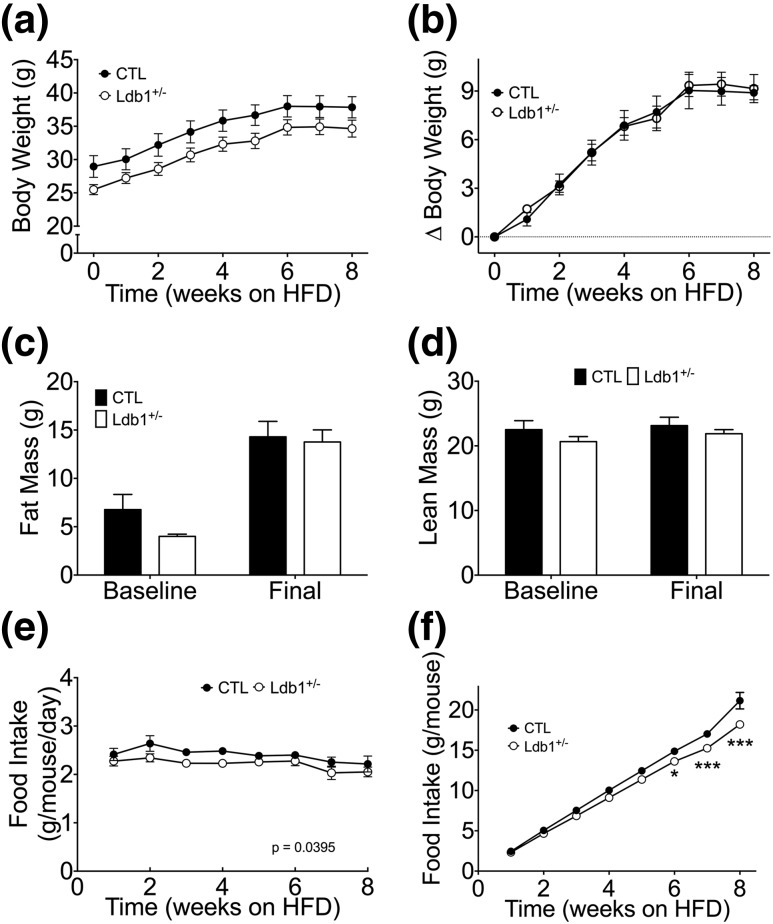
In light of our evidence that LDB1 is expressed across multiple metabolic tissues and influences insulin sensitivity [Fig. 1(a) and Fig. 2(a) and 2(b)], we hypothesized that LDB1 has roles beyond the β-cell to impact metabolism. To investigate this hypothesis, we assessed components of energy balance in Ldb1+/− mice during chronic HF feeding. Although Ldb1+/− mice were slightly smaller at the study onset, they displayed similar diet-induced body and fat mass accrual [Fig. 3(a–c)] and similar lean mass (before and after HF feeding) when compared with controls [Fig. 3(d)]. In spite of normal obesogenic response, Ldb1+/− mice were characterized by reduced average weekly food intake (P = 0.0395), resulting in diminished cumulative consumption by week 6 of HFD [Fig. 3(e) and 3(f)].
Figure 3.
Energy balance during HF feeding in Ldb1+/− and control (CTL) littermates. (a) Average weekly body weight. (b) Change in body weight from the onset of HF feeding. (c) Fat mass. (d) Lean mass. (e) Average weekly food intake. (f) Cumulative food intake throughout the study period. Data represented as mean ± SEM. *P < 0.05; ***P < 0.001; n = 10 to 14 mice/group.
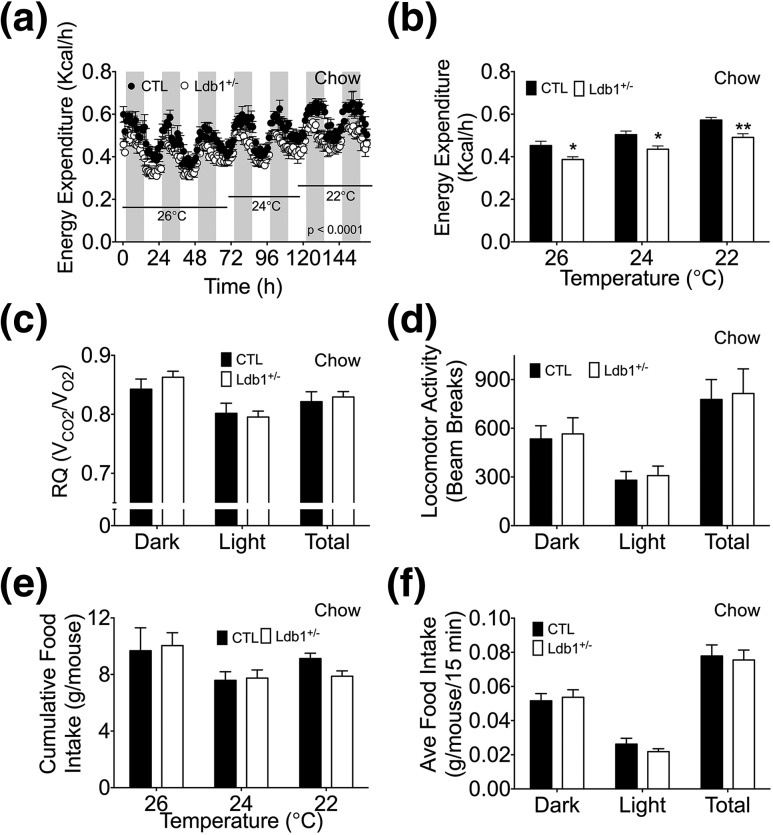
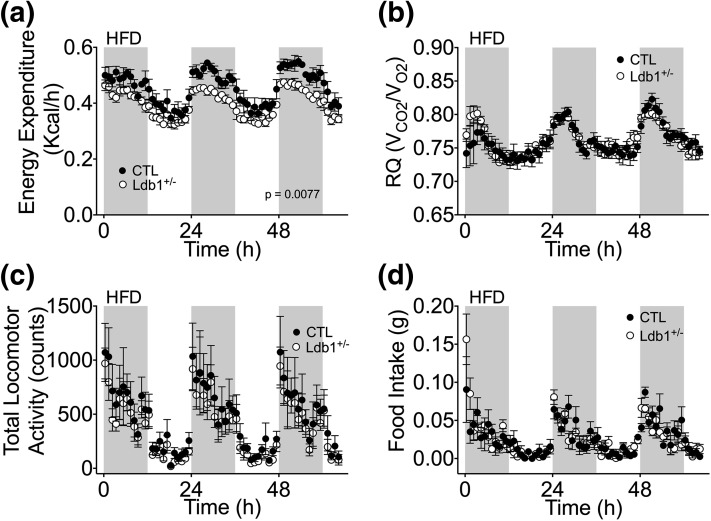
Because of the apparent energy imbalance, we hypothesized that EE is impaired in these mice. Using indirect calorimetry, we identified reduced EE in Ldb1+/− mice that could not be rescued by decreasing the ambient temperature [Fig. 4(A) and 4(B); Supplemental Fig. 3(a–c) (68.1MB, tiff) ]. Analysis of EE by analysis of covariants (19) identified an expected correlation with body weight (Supplemental Fig. 4 (42.2MB, tiff) ); however, based on our sample size, we also analyzed this data utilizing nonparametric methodology and uncovered an association (P = 0.0498) between genotype and EE when adjusted for body weight (Supplemental Fig. 4 (42.2MB, tiff) ). Importantly, reduced EE was also observed during HF feeding [Fig. 5(a)]. Although EE was suppressed, substrate utilization (RQ) and locomotor activity were similar in control and Ldb1+/− mice, regardless of diet [Fig. 4(c) and 4(d) and Fig. 5(b) and 5(c); Supplemental Fig. 3(d) and 3(e) (68.1MB, tiff) ]. In contrast to our data obtained from long-term HF feeding, food intake during this 1-week study was comparable between control and Ldb1+/− mice [Fig. 4(e) and 4(f) and Fig. 5(d); Supplemental Fig. 3(f) (68.1MB, tiff) ]. It is important to note that beyond the contracted duration of HF feeding, the indirect calorimetry study also differed in that all mice were single housed, an experimental condition known to influence EE and food intake (19).
Figure 4.
Indirect calorimetry analysis of Ldb1+/− and control (CTL) littermates. (a, b) EE (kcal/h) at 26°C, 24°C, and 22°C in chow-fed mice. (c) Diurnal patterns of substrate utilization [RQ (VCO2/VO2)] at 26°C. (d) Locomotor activity (counts) at 26°C. (e) Cumulative food intake (g/mouse) at 26°C, 24°C, and 22°C. (f) Diurnal patterns of average food intake (g/mouse/15 min) at 26°C. Data represented as mean ± SEM. *P < 0.05; **P < 0.01; n = 5 mice/group.
Figure 5.
Indirect calorimetry analysis of Ldb1+/− and control (CTL) littermates on HFD at 26°C. (a) Circadian EE (kcal/h). (b) RQ (VCO2/VO2). (c) Total locomotor activity (counts). (d) Food intake (g/mouse). Data represented as mean ± SEM (n = 5 mice/group).
LDB1 regulates BAT gene expression
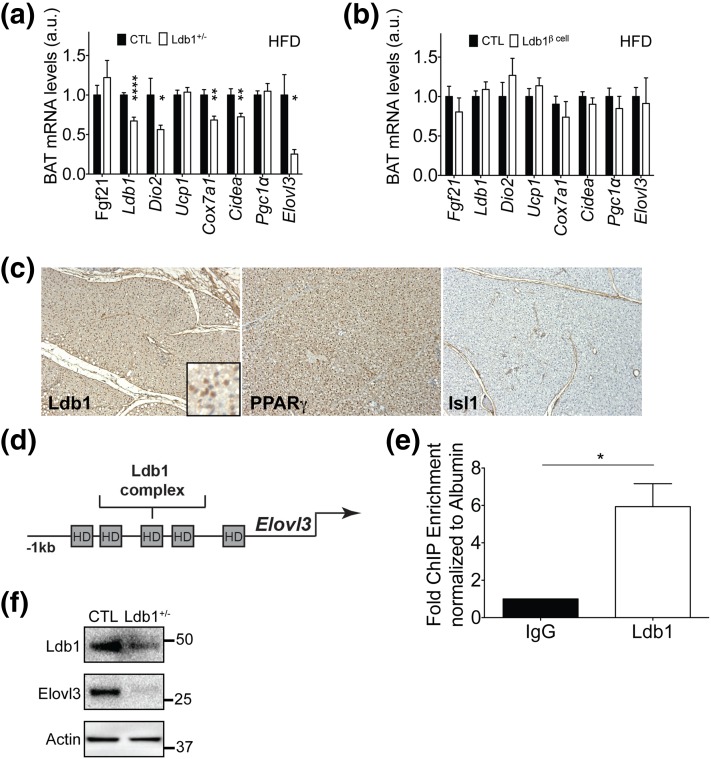
The indirect calorimetry results suggested LDB1 regulates not only EE, but also the thermogenic response to cold exposure. In rodents, BAT has important functions in thermo-regulation and energy homeostasis (20, 21). Therefore, we analyzed mRNA levels of BAT thermogenic genes to determine whether HF-fed Ldb1+/− mice have reduced EE potential. Expression of several BAT markers and genes relating to BAT oxidative metabolism and mitochondrial biogenesis were decreased in Ldb1+/− mice, including cell death activator CIDE-A (Cidea), Elongase of very long-chain fatty acids (Elovl3), and Cytochrome c oxidase 7a1 (Cox7a1) [Fig. 6(a)], suggesting that the BAT program may be sensitive to Ldb1 gene dosage. We also observed a 50% reduction in BAT expression of Dio2 in HF-fed Ldb1+/− mice [Fig. 6(a)], an enzyme crucial in BAT response to sympathetic tone (22).
Figure 6.
BAT-expressed LDB1 regulates key thermogenic factors. BAT expression analysis of gluco- and lipo-regulatory genes and thermogenic genes in (a) Ldb1+/− (n = 10 to 14 mice/group) and (b) Ldb1Δβ-cell (n= 6 to 13 mice/group) compared with their respective control (CTL) littermates after HF feeding. BAT mRNA levels were normalized to Hprt or 36B4. (c) Immunohistochemical staining images for LDB1, PPARγ, and ISL1 in BAT from chow-fed CTL C57BL/6 mice. (d) Schematic of the mouse Elovl3 5′ sequence demonstrating several putative core homeodomain-binding elements that may associate with LDB1 complexes. (e) LDB1 ChIP using chromatin isolated from chow-fed CTL mouse BAT demonstrates LDB1 enrichment of Elovl3 5′ sequences containing a homeodomain binding element (site 3 in d; n = 4). (f) Representative Western-blot image of LDB1 and Elovl3 protein in BAT of chow-fed CTL and Ldb1+/− mice (n = 3 for each genotype). Actin is included as loading control. Protein markers (in kilodaltons) are shown. Data are represented as mean ± SEM. *P < 0.05; **P < 0.01; ****P < 0.0001. a.u., arbitrary units.
We recognized the possibility that changes in BAT mRNA levels could be an indirect effect of the reduced insulin in the Ldb1+/− mice. To assess this, we performed a parallel analysis of the impact of HF feeding on mice in which LDB1 was inducibly deleted in postnatal pancreatic β-cells [termed Ldb1Δβ-cell; Supplemental Fig. 5(a) (72.2MB, tiff) ]. In agreement with the findings of Ediger et al. (11), Ldb1Δβ-cell mice exhibit glucose intolerance (data not shown) and markedly impaired circulating insulin levels [Supplemental Fig. 5(b) (72.2MB, tiff) ] (11). Strikingly, Ldb1Δβ-cell mice exhibit normal food intake and energy balance [Supplemental Fig. 5(c–e) (72.2MB, tiff) and Supplemental Fig. 6 (39.3MB, tiff) ], and analysis of BAT gene expression in Ldb1Δβ-cell mice identified no overt changes in the expression of critical thermogenic genes [Fig. 6(b)]. Together these data strongly argue that the alterations in BAT gene expression in the global Ldb1+/− mice are not a consequence of reduced plasma insulin.
These findings raised the intriguing possibility that LDB1, either directly or secondary to its role in other tissues, regulates BAT gene programming. Consistent with a cell-autonomous role, we observed Ldb1 transcript and Ldb1 immunoreactivity in BAT [Fig. 2(d) and Fig. 6(a) and 6(c)]. In agreement with our expression analysis, we detected nuclear LDB1 signal in cells throughout the PPARɣ+ interscapular brown adipose depot [Fig. 6(c)]. Moreover, signal for ISL1, a direct-binding partner of LDB1, was absent [Fig. 6(c)], suggesting LDB1 is likely partnering with another LIM-HD or LMO factor in this tissue. Further, we performed an LDB1 ChIP using chromatin isolated from chow-fed C57BL/6 mouse BAT and then quantified enrichment of upstream sequences from the most affected mRNA in the Ldb1+/− mice, Elovl3 [Fig. 6(a) and 6(d)]. The sequence examined is located within chr19:46130819-46132067 and bears several TAAT/ATTA elements, suggestive of binding by LIM-HD transcription factors, one class of LDB1-interacting partners. As compared with IgG control, LDB1 significantly enriched for upstream 5′ Elovl3 sequences [Fig. 6(e)] in a promoter region also bound by PPARγ (23, 24). This indicates that BAT-expressed LDB1 directly regulates a metabolic gene pivotal in brown adipocyte function. In further support of LDB1-mediated regulation of Elovl3, we observed reduced Elovl3 protein levels after western-blotting BAT whole-cell extracts from chow-fed Ldb1+/− mice, as compared with controls [Fig. 6(f)]. Taken together, our findings strongly argue that LDB1 directly impacts BAT gene expression.
Discussion
The transcriptional coregulator LDB1 is known for its role in pancreatic islet development and function (9, 11), erythropoiesis (10), and spinal cord neural subtype specification (25). However, metabolic function in nonpancreatic tissues has yet to be elucidated. Moreover, LDB1 is broadly expressed, notably in various tissues involved in glucose and lipid homeostasis. Thus, our studies encompassed a global investigation of LDB1 to determine its impact on various aspects of metabolism. In summary, we uncovered LDB1 impacts on EE regulation of energy balance, insulin action, and excitingly unique roles in BAT. Although the increase in insulin sensitivity and decreased BAT function may initially appear inconsistent, given the broad expression of LDB1 in multiple metabolic tissues (e.g., BAT, white adipose tissue, liver, skeletal muscle, and brain), we hypothesize that LDB1 has tissue-specific roles that may differentially impact (for example) insulin sensitivity in liver and muscle, while decreasing activity in BAT.
Ldb1+/− mice subjected to indirect calorimetry analysis revealed an overall reduced EE. Notably, during this EE analysis, we did not observe differences in food intake. Although this may be initially interpreted as an inconsistency between the studies, there are key variables that must be considered. First, our initial observations involved mice during chronic HF feeding, although the latter were made in a study lasting just 3 days. Secondly, mice in the chronic HF feeding study were group housed, while feeding measured during the calorimetry study occurred during single housing. With group-housed mice, confounding factors such as food spilling, social dominance, and aggression can dramatically influence traits linked to energy balance (19). Additionally, group-housed mice often huddle, which considerably reduces thermoregulatory requirements and thus EE and intake (19). Findings published during study describe the expression of human growth hormone (hGH) in the MIP-CreERT model (26) and bring to question the role growth hormone has on EE and its implications in the interpretation of our data. However, Heffernan et al. reported that inducing pharmacological levels of hGH failed to alter EE in lean or obese mice (27). Our findings are further bolstered by the observation that the hGH produced by a MIP-FoxM1-hGH model was undetectable in plasma (28), and together suggest that MIP-CreERT-derived hGH has no impact on EE.
Because BAT thermogenesis is a primary component of EE in mice (29, 30), we hypothesized that LDB1 is also a critical regulator of BAT function. In support of this hypothesis, essential BAT mRNAs, including those related to mitochondrial and BAT function, were suppressed. Specifically, we observed reduced mRNA in DIO Ldb1+/− mice encoding for the lipid droplet protein Cidea (an established marker of BAT in rodents) (31), the oxidative protein Cox7a1, and Elovl3, which is necessary for synthesis of very long-chain fatty acids and full metabolic capacity in brown adipocytes (11). The reduction of these gene products in this study does not appear to be linked to expression of the transcriptional coactivator Pgc1α (32), as mRNA levels were unchanged. We also found Ldb1 occupying the 5′ promoter region of Elovl3 by ChIP, providing evidence for direct LDB1 action in BAT.
We also identified a reduction in the mRNA encoding DIO2, an enzyme required for rodent BAT EE (22). Dio2 induction in BAT stimulates the conversion of intracellular thyroxine (T4) to 3,3′,5-triiodothyronine (T3), enhancing thermogenic responses (33). Thus, reduced DIO2 might indicate that BAT is less responsive to the major activating signals for thermogenesis. BAT is densely innervated and regulated by the sympathetic nervous system (34, 35). Sympathetic outflow to BAT increases norepinephrine release from postganglionic nerve terminals that bind to brown adipocyte β3-adrenoceptors (36), ultimately activating UCP1 activity, EE, and thermogenic gene expression (20). Taken together with evidence demonstrating that DIO2 is pivotal in increasing adrenergic responsiveness (37, 38), our findings suggest that reduced expression of Dio2 in Ldb1+/− mice may interfere with sympathetic nervous system–induced activation of BAT EE. Plasma analyses revealed significantly elevated T3 and T4 in the Ldb1+/− DIO cohort, as compared with DIO controls (Supplemental Table 2 (29.5KB, pdf) ). Although a reduction in DIO2 may account for increased T4, there is no clear mechanism by which it would also increase T3. Thus, although it is tempting to associate this with changes in BAT Dio2 mRNA, we do not yet know if the Ldb1+/− mice also have defects in the hypothalamic-pituitary-thyroid axis or governing feedback mechanisms. It is interesting to note that LDB1 has roles in the pituitary (39) and human genome-wide association studies have linked elevated T4 and thyroid-stimulating hormone to the pituitary LIM-HD transcription factor (and Ldb1 partner) LHX3 (39). Future studies will dissect the contributions of BAT- vs hypothalamic-pituitary-thyroid–expressed LDB1 in EE control.
LDB1 is a scaffolding coregulator lacking DNA-binding and direct gene activation/repression capacity (40). Rather it has high avidity for the LIM-domain family of proteins (including LIM-HD and LMO factors) (41, 42), including the ISL1 transcription factor. ISL1 is required for islet development and postnatal β-cell function (43–45); however, it is unclear which LIM factors mediate activity in other LDB1-sensitive tissues such as BAT, as revealed in our study. We did not observe ISL1 expression in BAT, raising the likelihood that other LMO or LIM-HD partners mediate the effect of LDB1 on BAT gene expression. ChIP revealed LDB1 enrichment of the Elovl3 promoter in a domain enriched in LIM-HD binding TAAT/ATTA elements. The related LIM-HD factor LHX8 is 1 candidate for mediating LDB1 effects in the BAT, as it is a brown adipose–specific marker (8, 46). However, we cannot exclude the possibility that LDB1 interacts with LMO-mediated Gata and basic helix-loop-helix transcription factor complexes in this tissue.
Overall, our study strongly supports insulin-independent roles for LDB1 in the regulation of energy homeostasis during DIO, in part through direct effects on BAT gene expression. Future studies will aim to identify the tissue-specific action of LDB1 in regulation of energy homeostasis.
Acknowledgments
We thank Jeff Ishibashi and Patrick Seale (University of Pennsylvania) for advice with the BAT ChIP experiments.
Acknowledgments
This work was supported by National Institutes of Health Grants DRC P30 DK079626 and DK094842 (to C.S.H.), ADA 1-16-JDF-044 (to C.S.H.), DK098319 (to K.M.H.), AR062128 (to G.C.R.), HL123574 (to M.Y.), 1T32 HD071866 (to C.L.), and DK068157 (to D.A.S.). The University of Alabama at Birmingham Small Animal Phenotyping Core used in these studies was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK056336. Research reported in this manuscript was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001417.
Author contributions: C.L., D.A.S., G.C.R., M.Y., K.M.H., and C.S.H. were responsible for study conception and design. C.L., Y.L., T.K., C.H., J.G., M.B., B.N.E., Y.T., G.C.R., M.Y., C.S., K.M.H., and C.S.H. generated experimental data. C.L., D.A.S., G.C.R., M.Y., K.M.H., and C.S.H. were responsible for data analysis and interpretation. C.L., B.N.E., D.A.S., G.C.R., K.M.H., and C.S.H. were responsible for drafting and critical review of the manuscript. T.A.S. was responsible for statistical analysis of the data. C.S.H and K.M.H. are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- ChIP
- chromatin immunoprecipitation
- Cidea
- Cell death activator CIDE-A
- Cox7a1
- Cytochrome c oxidase 7a1
- DIO
- diet-induced obesity
- Dio2
- diodinase type 2
- EE
- energy expenditure
- HFD
- high-fat diet
- hGH
- human growth hormone
- IgG
- immunoglobulin G
- IP
- intraperitoneal
- LIM
- Lin11, Isl1, Mec3; LMO, LIM-only
- mRNA
- messenger RNA
- PCR
- polymerase chain reaction
- PPARγ
- proliferator-activated receptor-γ
- RQ
- respiratory quotient
- SEM
- standard error of the mean
- Ucp1
- uncoupling protein 1.
References
- 1.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandviwala T, Khalid U, Deswal A. Obesity and cardiovascular disease: a risk factor or a risk marker? Curr Atheroscler Rep. 2016;18(5):21. [DOI] [PubMed] [Google Scholar]
- 3.Farmer SR. Molecular determinants of brown adipocyte formation and function. Genes Dev. 2008;22(10):1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koppen A, Kalkhoven E. Brown vs white adipocytes: the PPARgamma coregulator story. FEBS Lett. 2010;584(15):3250–3259. [DOI] [PubMed] [Google Scholar]
- 5.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4(4):611–617. [DOI] [PubMed] [Google Scholar]
- 6.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4(4):585–595. [DOI] [PubMed] [Google Scholar]
- 7.Sears IB, MacGinnitie MA, Kovacs LG, Graves RA. Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 1996;16(7):3410–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6(1):38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter CS, Dixit S, Cohen T, Ediger B, Wilcox C, Ferreira M, Westphal H, Stein R, May CL. Islet α-, β-, and δ-cell development is controlled by the Ldb1 coregulator, acting primarily with the Islet-1 transcription factor. Diabetes. 2013;62(3):875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love PE, Warzecha C, Li L. Ldb1 complexes: the new master regulators of erythroid gene transcription. Trends Genet. 2014;30(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ediger BN, Lim HW, Juliana C, Groff DN, Williams LT, Dominguez G, Liu JH, Taylor BL, Walp ER, Kameswaran V, Yang J, Liu C, Hunter CS, Kaestner KH, Naji A, Li C, Sander M, Stein R, Sussel L, Won KJ, May CL, Stoffers DA. LIM domain-binding 1 maintains the terminally differentiated state of pancreatic β cells. J Clin Invest. 2017;127(1):215–229. [DOI] [PMC free article] [PubMed]
- 12.Westerberg R, Månsson JE, Golozoubova V, Shabalina IG, Backlund EC, Tvrdik P, Retterstøl K, Capecchi MR, Jacobsson A. ELOVL3 is an important component for early onset of lipid recruitment in brown adipose tissue. J Biol Chem. 2006;281(8):4958–4968. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay M, Teufel A, Yamashita T, Agulnick AD, Chen L, Downs KM, Schindler A, Grinberg A, Huang SP, Dorward D, Westphal H. Functional ablation of the mouse Ldb1 gene results in severe patterning defects during gastrulation. Development. 2003;130(3):495–505. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Kwan KM, Mailloux CM, Lee WK, Grinberg A, Wurst W, Behringer RR, Westphal H. LIM-homeodomain proteins Lhx1 and Lhx5, and their cofactor Ldb1, control Purkinje cell differentiation in the developing cerebellum. Proc Natl Acad Sci USA. 2007;104(32):13182–13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamarina NA, Roe MW, Philipson L. Characterization of mice expressing Ins1 gene promoter driven CreERT recombinase for conditional gene deletion in pancreatic β-cells. Islets. 2014;6(1):e27685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habegger KM, Stemmer K, Cheng C, Müller TD, Heppner KM, Ottaway N, Holland J, Hembree JL, Smiley D, Gelfanov V, Krishna R, Arafat AM, Konkar A, Belli S, Kapps M, Woods SC, Hofmann SM, D’Alessio D, Pfluger PT, Perez-Tilve D, Seeley RJ, Konishi M, Itoh N, Kharitonenkov A, Spranger J, DiMarchi RD, Tschöp MH. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62(5):1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harms MJ, Lim HW, Ho Y, Shapira SN, Ishibashi J, Rajakumari S, Steger DJ, Lazar MA, Won KJ, Seale P. PRDM16 binds MED1 and controls chromatin architecture to determine a brown fat transcriptional program. Genes Dev. 2015;29(3):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenna B, Guo M, Reynolds A, Hara M, Stein R. Dynamic recruitment of functionally distinct Swi/Snf chromatin remodeling complexes modulates Pdx1 activity in islet β cells. Cell Reports. 2015;10(12):2032–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschöp MH, Speakman JR, Arch JR, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 21.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404(6778):652–660. [DOI] [PubMed] [Google Scholar]
- 22.de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108(9):1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4(4):263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi T, Fujimori K. Very long-chain-fatty acids enhance adipogenesis through coregulation of Elovl3 and PPARγ in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2012;302(12):E1461–E1471. [DOI] [PubMed] [Google Scholar]
- 25.Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110(2):237–249. [DOI] [PubMed] [Google Scholar]
- 26.Oropeza D, Jouvet N, Budry L, Campbell JE, Bouyakdan K, Lacombe J, Perron G, Bergeron V, Neuman JC, Brar HK, Fenske RJ, Meunier C, Sczelecki S, Kimple ME, Drucker DJ, Screaton RA, Poitout V, Ferron M, Alquier T, Estall JL. Phenotypic characterization of MIP-CreERT1Lphi mice with transgene-driven islet expression of human growth hormone. Diabetes. 2015;64(11):3798–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heffernan MA, Thorburn AW, Fam B, Summers R, Conway-Campbell B, Waters MJ, Ng FM. Increase of fat oxidation and weight loss in obese mice caused by chronic treatment with human growth hormone or a modified C-terminal fragment. Int J Obes Relat Metab Disord. 2001;25:1442–1449. [DOI] [PubMed]
- 28.Baan M, Kibbe CR, Bushkofsky JR, Harris TW, Sherman DS, Davis DB. Transgenic expression of the human growth hormone minigene promotes pancreatic β-cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2015;309(7):R788–R794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwa JJ, Fawzi AB, Graziano MP, Ghibaudi L, Williams P, Van Heek M, Davis H, Rudinski M, Sybertz E, Strader CD. Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am J Physiol. 1997;272(4 Pt 2):R1204–R1209. [DOI] [PubMed] [Google Scholar]
- 30.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281(5726):31–35. [DOI] [PubMed] [Google Scholar]
- 31.Li P. Cidea, brown fat and obesity. Mech Ageing Dev. 2004;125(4):337–338. [DOI] [PubMed] [Google Scholar]
- 32.Hallberg M, Morganstein DL, Kiskinis E, Shah K, Kralli A, Dilworth SM, White R, Parker MG, Christian M. A functional interaction between RIP140 and PGC-1alpha regulates the expression of the lipid droplet protein CIDEA. Mol Cell Biol. 2008;28(22):6785–6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva JE, Larsen PR. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature. 1983;305(5936):712–713. [DOI] [PubMed] [Google Scholar]
- 34.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes. 2010;34(Suppl 1):S36–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richard D, Picard F. Brown fat biology and thermogenesis. Front Biosci (Landmark Ed). 2011;16:1233–1260. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Unelius L, Bengtsson T, Cannon B, Nedergaard J. Coexisting beta-adrenoceptor subtypes: significance for thermogenic process in brown fat cells. Am J Physiol. 1994;267(4 Pt 1):C969–C979. [DOI] [PubMed] [Google Scholar]
- 37.Rubio A, Raasmaja A, Maia AL, Kim KR, Silva JE. Effects of thyroid hormone on norepinephrine signaling in brown adipose tissue. I. Beta 1- and beta 2-adrenergic receptors and cyclic adenosine 3′,5′-monophosphate generation. Endocrinology. 1995;136(8):3267–3276. [DOI] [PubMed] [Google Scholar]
- 38.Rubio A, Raasmaja A, Silva JE. Thyroid hormone and norepinephrine signaling in brown adipose tissue. II: Differential effects of thyroid hormone on beta 3-adrenergic receptors in brown and white adipose tissue. Endocrinology. 1995;136(8):3277–3284. [DOI] [PubMed] [Google Scholar]
- 39.Bach I, Carrière C, Ostendorff HP, Andersen B, Rosenfeld MG. A family of LIM domain-associated cofactors confer transcriptional synergism between LIM and Otx homeodomain proteins. Genes Dev. 1997;11(11):1370–1380. [DOI] [PubMed] [Google Scholar]
- 40.Matthews JM, Visvader JE. LIM-domain-binding protein 1: a multifunctional cofactor that interacts with diverse proteins. EMBO Rep. 2003;4(12):1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bach I. The LIM domain: regulation by association. Mech Dev. 2000;91(1-2):5–17. [DOI] [PubMed] [Google Scholar]
- 42.Hunter CS, Rhodes SJ. LIM-homeodomain genes in mammalian development and human disease. Mol Biol Rep. 2005;32(2):67–77. [DOI] [PubMed] [Google Scholar]
- 43.Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385(6613):257–260. [DOI] [PubMed] [Google Scholar]
- 44.Du A, Hunter CS, Murray J, Noble D, Cai CL, Evans SM, Stein R, May CL. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes. 2009;58(9):2059–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ediger BN, Du A, Liu J, Hunter CS, Walp ER, Schug J, Kaestner KH, Stein R, Stoffers DA, May CL. Islet-1 Is essential for pancreatic β-cell function. Diabetes. 2014;63(12):4206–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J, Cannon B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA. 2007;104(11):4401–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]