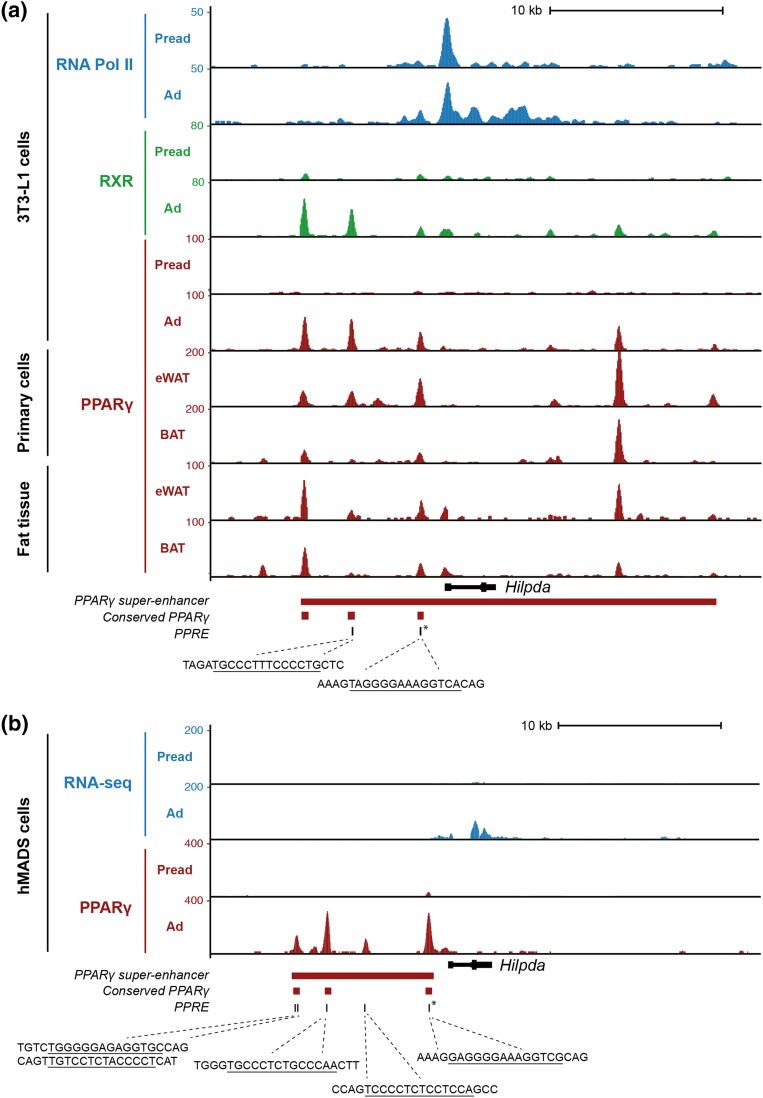
Figure 3.
PPARγ is associated with the HILPDA locus in mouse and human adipocytes. (a) Screenshot of the mouse Hilpda locus showing ChIP-seq profiles of retinoid X receptor (RXR), PPARγ, and RNA polymerase II in 3T3-L1 preadipocytes and adipocytes, and PPARγ ChIP-seq profiles from eWAT and BAT (primary adipocytes and whole tissue). (b) Screenshot of the human HILPDA locus showing RNA sequencing (RNA-seq) and PPARγ ChIP-seq profiles in hMADS preadipocytes and adipocytes. PPARγ binding sites that are conserved between mouse and human and the position of PPAR response elements within the PPARγ binding sites are highlighted. The sequences indicate conserved PPAR response elements (PPREs) in the respective binding sites. The red bar indicates the position of adipocyte-specific PPARγ superenhancers in 3T3-L1 and hMADS adipocytes. The PPARγ binding site that has also been reported to bind PPARα in liver and that harbors a highly conserved and experimentally confirmed PPAR response element (16) is marked by an asterisk. Ad, adipocytes; Pread, preadipocytes.