Figure 1.
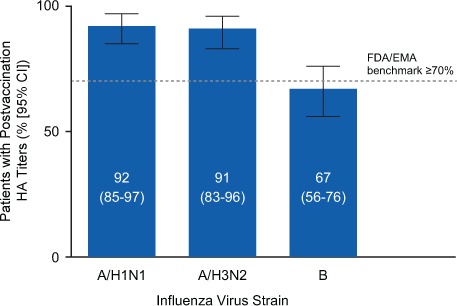
Proportion of the 90 substudy patients achieving seroprotection
Seroprotection was defined as a postvaccination anti–hemagglutination immunoglobulin G titer of 40 or higher according to US Food and Drug Administration (FDA) and European Medicines Agency (EMA) seroprotection benchmark guidelines (≥70%).12,13 A/H1N1, A/California/7/2009; A/H3N2, A/Texas/50/2012; B, B/Massachusetts/2/2012; CI, confidence interval.
