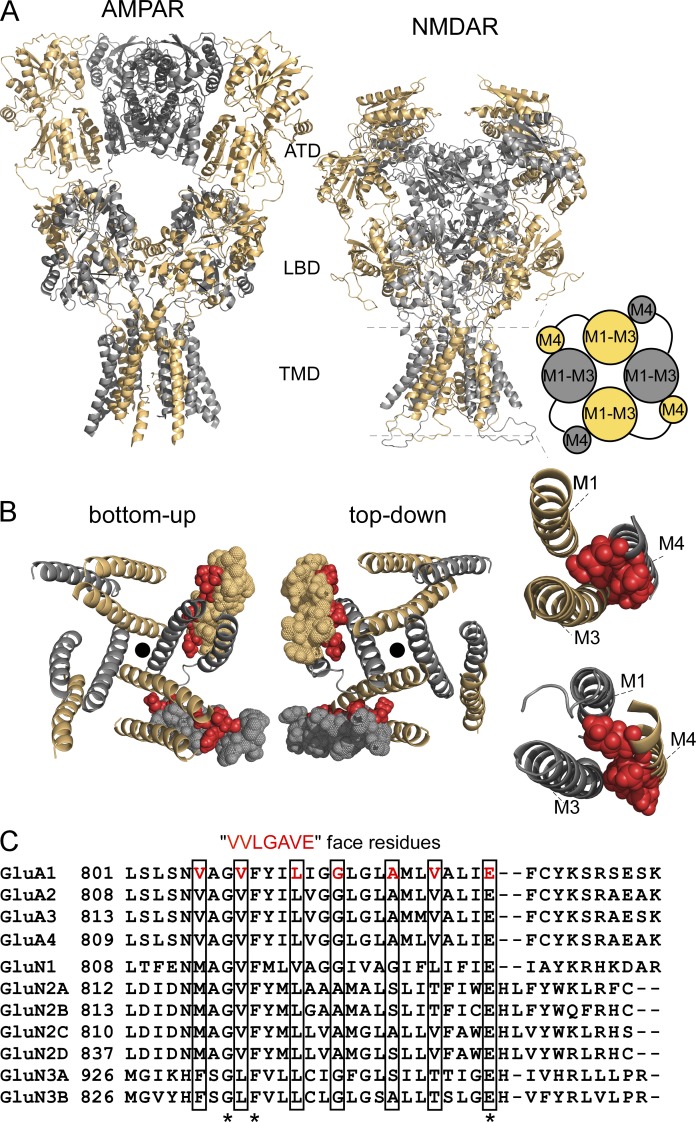
Figure 1.
Structural features of AMPAR and NMDAR TMDs. (A) Comparison of AMPARs (GluA2, 3KG2, Sobolevsky et al., 2009) and NMDARs (model structure based on 4TLM of GluN1/GluN2B, see Materials and methods) lacking the intracellular CTD. Subunits are colored light orange (GluA2 A and C, GluN1) and gray 60% (GluA2 B and D, GluN2A). For iGluRs, individual subunits as well as the oligomeric complexes are composed of four highly modular domains. Two of these domains are positioned on the extracellular side of the membrane: the amino-terminal domain (ATD) and the LBD. The TMD spans the lipid bilayer and forms the ion channel; in both receptor subtypes at the level of the TMD, the M4 segment of one subunit is associated with the pore domain or ion channel core (M1–M3) of a neighboring subunit (cartoon, right). The fourth domain is the intracellular CTD. (B) View of the TMD for the model NMDAR structure from either the intracellular (bottom-up view) or extracellular (top-down view) side illustrating the association of M4 with an adjacent pore domain. For clarity, one GluN1 and one GluN2A M4 segment are represented as spheres, with VVLGAVE face positions highlighted in red. The center of the pore is indicated by a black dot. (far right) Illustration showing that residues occupying the VVLGAVE face in NMDAR subunits are aligned quite closely, mainly with the M3 segment of an adjacent subunit. The S1-M1 linker of the same subunit is also positioned closely to the extracellular portion of its M4 segments (not depicted). (C) Alignment of the M4 segments and residues on the N- and C-terminal sides for rat AMPAR and NMDAR subunits. Only three residues, a glycine (G), phenylalanine (F), and a glutamate (E), are completely conserved across all subunits (asterisks). Still, residues occupying the VVLGAVE face (boxed) tend to have comparable noncharged (valine [V], leucine [L], methionine [M]) or small (glycine [G], alanine [A], serine [S]) side chains with the exception of a threonine (T) at the VVLGAVE position.