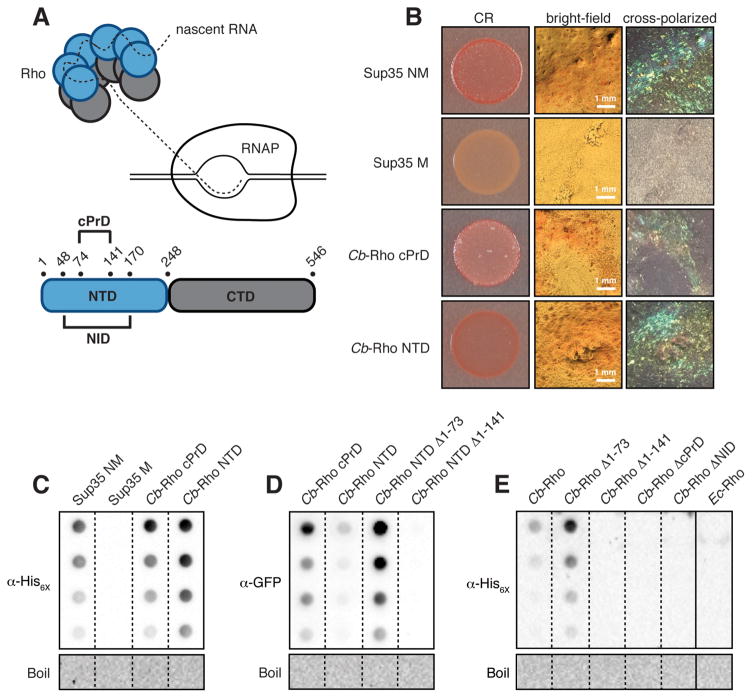
Figure 1.
Cb-Rho contains an amyloidogenic cPrD. (A) (Top) Cartoon of a Rho hexamer engaging RNAP. (Bottom) Domain organization of Cb-Rho highlighting its cPrD and NID. (B) E. coli cells exporting the indicated protein domain were spotted on solid medium containing the amyloid-binding dye Congo Red (CR). The PrD of yeast Sup35 (NM) and its derivative (M) serve as positive and negative controls, respectively. Plate-derived material visualized by bright-field microscopy and between crossed polarizers reveals “apple-green” birefringence characteristically exhibited by CR-bound amyloid aggregates. (C) Amyloid-like aggregates (characterized by their resistance to denaturation in SDS) were detected in plate-derived material from (B) as assessed by filter-retention analysis. Undiluted sample and three two-fold dilutions are shown. Aggregates were no longer detected once boiled (see Supplementary Materials). (D) SDS-stable aggregates were detected in cell lysates containing the indicated cPrD-containing Cb-Rho fragment fused to mYFP, but not a cPrD-lacking variant. (E) SDS-stable aggregates were detected in cell lysates containing hexahistidine-tagged, cPrD-containing Cb-Rho, but not cPrD-lacking variants or Ec-Rho. (C–E) See Fig S3 for immunoblot analyses.