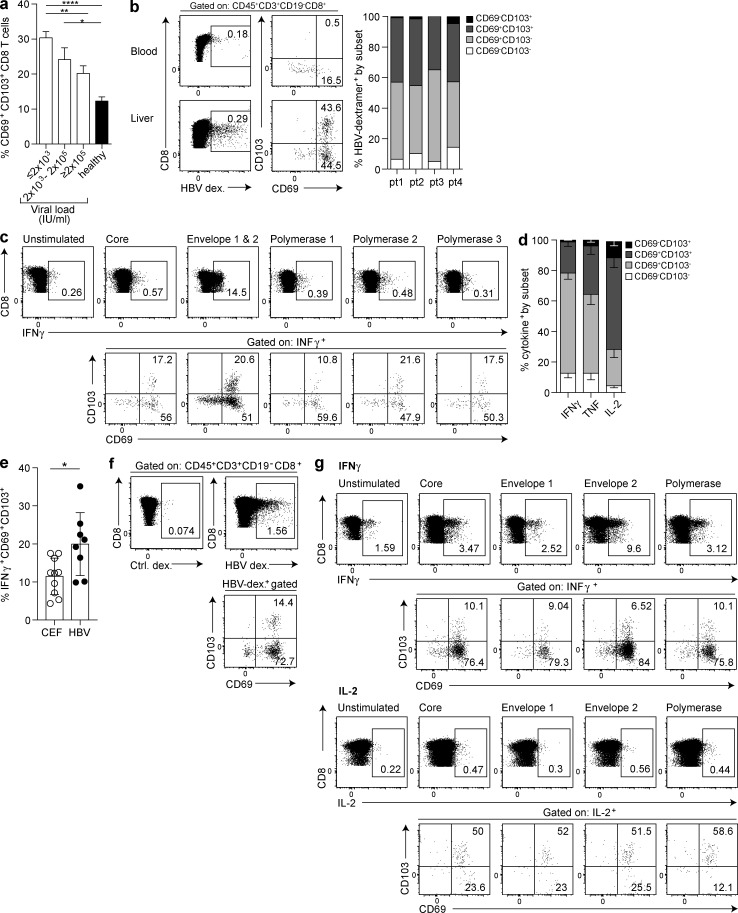
Figure 4.
Liver CD8 TRM include virus-specific CD8 and are associated with HBV control. (a) Frequencies of CD45RA−CD69+CD103+ CD8 T cells in HBV-infected liver biopsies stratified by viral load (IU/ml; ≤2,000, n = 10; 2 × 103 − 2 × 105, n = 15; >2 × 105, n = 8; all treatment naive) compared with healthy livers (n = 54). (b–d) Proportion of HBV-specific CD8 T cells in CHB expressing residency markers CD103 and CD69, identified by ex vivo staining with a panel of HLA-A2–HBV peptide dextramers (example plots for blood and an HBV+ resection liver; summary data n = four livers; one resection, three biopsies; b) or after stimulation of IHL for 16 h with 10 µg/ml of overlapping peptides spanning core, polymerase, or envelope regions (using an HBsAg+ HBV perfusate; c) and after 16-h stimulation with core (HBV genotype D) alone and intracellular cytokine staining for IFNγ, TNF, and IL-2 (n = 7; d). (e) Proportion of CEF–specific (n = 10; four margins, six biopsies) and HBV-specific (n = 8; one resection, one perfusate, six biopsies) IFNγ CD8 responses expressing a CD45RA−CD69+CD103+ phenotype. (f) Detection of CD8 T cells according to CD69 and CD103 expression within ex vivo HLA-A2–HBV dextramer (dex.) panel-binding cells in an HLA-A2+ perfusate sample from an individual with HBsAg-resolved HBV infection. (g) IHLs from an HLA-A2 perfusate from an individual with HBsAg-resolved HBV infection were stimulated with 10 µg/ml of overlapping peptides spanning core, envelope, or polymerase regions of HBV genotype D for 16 h followed by intracellular cytokine staining for IFNγ and IL-2 and assessment of residency markers on cytokine-positive populations. Error bars indicate means ± SEM; *, P < 0.05; **, P < 0.01; ****, P < 0.0001; p-values were determined by a Kruskal-Wallis test (ANOVA) with a Dunn’s post hoc test for pairwise multiple comparisons (a) or Wilcoxon Signed-rank t test (e).