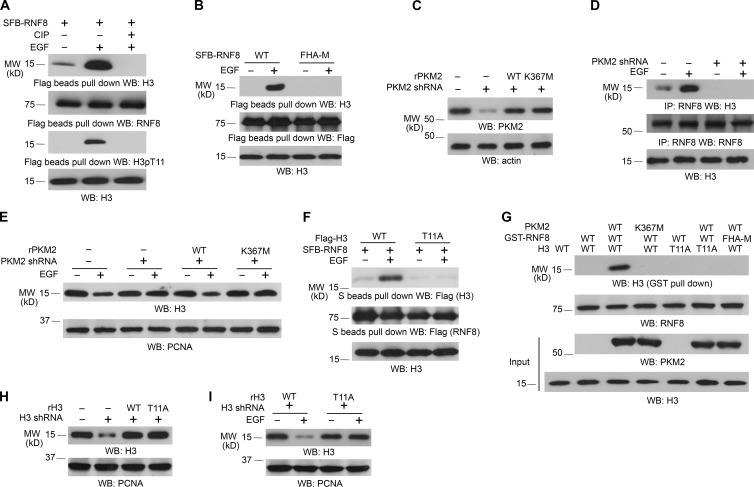
Figure 2.
RNF8 FHA domain binds to phosphorylated histone H3 at T11. Immunoblotting and immunoprecipitation (IP) analyses were performed with the indicated antibodies. WB, Western blot. (A) SFB-RNF8 immunoprecipitated from U251 cells with or without 100 ng/ml EGF treatment for 6 h was incubated with or without 10 U calf intestinal alkaline phosphatase (CIP) for 1 h at 37°C, followed by washing three times with PBS. LS-soluble chromatin extracts were prepared. (B) U251 cells expressing SFB-tagged WT RNF8 or RNF8 FHA-M mutant were treated with or without 100 ng/ml EGF for 6 h. LS-soluble chromatin extracts were prepared. (C–E) U251 cells with or without expression of PKM2 shRNA were reconstituted with the expression of WT rPKM2 or rPKM2 K367M mutant (C). Cells were treated with or without 100 ng/ml EGF for 6 h. Coimmunoprecipitation between histone H3 and RNF8 was examined (D). Histone H3 expression levels of cells treated with or without 100 ng/ml EGF for 24 h were examined (E). LS-soluble chromatin extracts were prepared (D and E). (F) U251 cells expressing SFB-tagged RNF8 and Flag-tagged WT histone H3.3B or H3.3B-T11A mutant were treated with or without 100 ng/ml EGF for 6 h. LS-soluble chromatin extracts were prepared. (G) Purified WT histone H3.3B or H3.3B-T11A mutant was mixed with His-PKM2 WT or His-PKM2 K367M for an in vitro phosphorylation reaction. The mixture was then incubated with purified and immobilized WT GST-RNF8 or GST-RNF8 FHA-M mutant on glutathione agarose beads for in vitro pull-down analyses. (H and I) U251 cells with or without expression of histone H3.3 shRNA and reconstituted expression of WT histone rH3.3B and rH3.3B-T11A mutant (H) were treated with or without 100 ng/ml EGF for 24 h (I). LS-soluble chromatin extracts were prepared. All of the experiments were repeated three times.