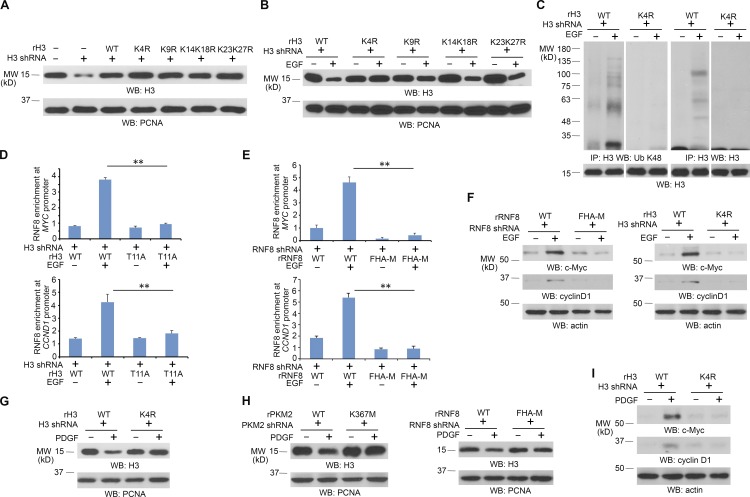
Figure 3.
RNF8-mediated histone H3 ubiquitylation at K4 promotes MYC and CCND1 expression. Immunoblotting analyses were performed with the indicated antibodies. IP, immunoprecipitation; WB, Western blot. (A and B) U251 cells expressing histone H3.3 shRNA were reconstituted with expression of WT rH3.3B or the indicated rH3.3B mutants (A). These cells were treated with or without 100 ng/ml EGF for 24 h (B). LS-soluble chromatin extracts were prepared. (C) U251 cells expressing histone H3.3 shRNA were reconstituted with expression of WT rH3.3B or rH3.3B K4R mutant. These cells were treated with or without 100 ng/ml EGF for 6 h in the presence of 25 µM MG132. Cell lysates for ubiquitylation assay were prepared. (D) Flag-RNF8 stably expressed U251 cells with histone H3.3 depletion and reconstituted expression of WT histone rH3.3B or rH3.3B-T11A were treated with or without 100 ng/ml EGF for 6 h. ChIP assays with an anti-Flag antibody and quantitative PCR analyses with primers against promoters of MYC and CCND1 were performed. (E) U251 cells with RNF8 depletion and reconstituted expression of WT rFlag-rRNF8 or rFlag-RNF8 FHA-M mutant were treated with or without 100 ng/ml EGF for 6 h. ChIP assay with an anti-Flag antibody and quantitative PCR analyses with primers against promoters of MYC and CCND1 were performed. (D and E) The data represent the means ± SD of three independent experiments. **, P < 0.001 by Student’s t test. (F) U251 cells with RNF8 depletion and reconstituted expression of WT rRNF8 or rRNF8 FHA-M mutant or with histone H3.3 depletion and reconstituted expression of WT histone rH3.3B or rH3.3B-K4R were treated with or without 100 ng/ml EGF for 24 h. Total cell lysates were prepared. (G) U251 cells with histone H3.3 depletion and reconstituted expression of WT histone rH3.3B or rH3.3B-K4R were treated with or without 100 ng/ml PDGF for 24 h. LS-soluble chromatin extracts were prepared. (H) U251 cells with PKM2 depletion and reconstituted expression of WT rPKM2 or rPKM2 K367M or with RNF8 depletion and reconstituted expression of WT rRNF8 or rRNF8 FHA-M mutant were treated with or without 100 ng/ml PDGF for 24 h. LS-soluble chromatin extracts were prepared. (I) U251 cells with histone H3.3 depletion and reconstituted expression of WT histone rH3.3B or rH3.3B-K4R were treated with or without 100 ng/ml PDGF for 24 h. Total cell lysates were prepared. All of the experiments were repeated three times.