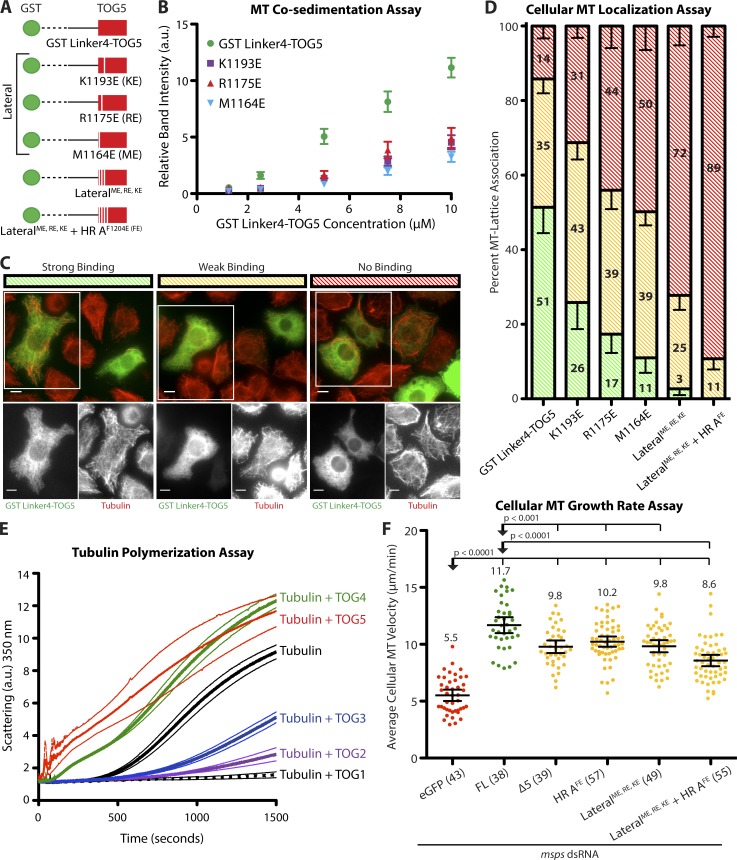
Figure 5.
TOG5 uses lateral tubulin-binding determinants to bind the MT lattice, nucleate MTs, and maintain cellular MT polymerization rates. (A) GST dimerized Linker4-TOG5 (1,085–1,411) constructs used for MT co-sedimentation and cellular MT localization assays. (B) GST Linker4-TOG5 co-sediments with taxol-stabilized MTs. Mutating K1193, R1175, or M1164 to glutamic acid reduces the apparent affinity of GST Linker4-TOG5 for the MT lattice. Mean ± SEM are shown (n = 5). (C) Representative immunofluorescence images of strong, weak, or no lattice binding in GST Linker4-TOG5 transfected S2 cells. Bars, 5 µm. (D) GST Linker4-TOG5 robustly associates with MTs in S2 cells. Mutating residues in HR 0, HR A, or HRs 0–A decreases the MT-lattice association activity of GST Linker4-TOG5. Mean ± SEM are shown (n = 3). (E) TOG domains differentially promote MT nucleation and polymerization in a light scattering assay. TOGs 1–3 sequester tubulin, increasing the lag time before MT nucleation and subsequent polymerization. In contrast, TOGs 4 and 5 decrease MT polymerization lag time. Mean ± SEM are shown (n = 3). (F) Mutating TOG5–MT binding determinants prevents full rescue of cellular MT polymerization rates. MT polymerization rates for the three mutants are similar to the rates obtained with the Msps Δ5 construct. Number of cells analyzed is shown in parentheses. Mean ± 95% confidence intervals are shown; two-tailed Mann–Whitney U test.