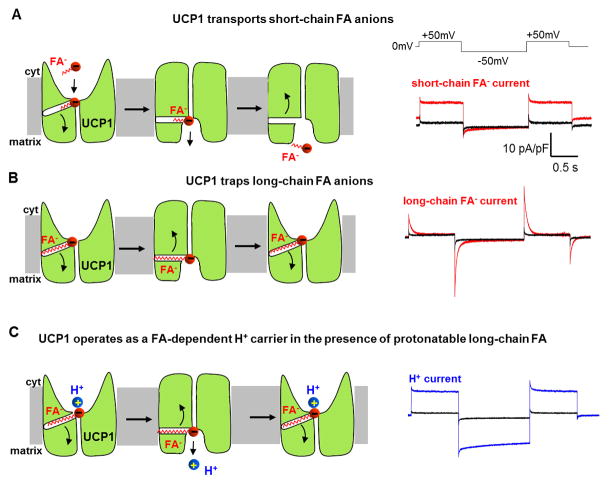
Figure 3. Model of UCP1 operation based on the electrophysiological data.
(A) Left panel: the mechanism of steady UCP1 current induced by short-chain low-pKa FA analogs added on the cytosolic face of the IMM. Short-chain FA are simply transported by UCP1 across the IMM. UCP1 has two conformation states, with substrate binding site exposed either to the cytosolic (c-state) or matrix (m-state) side of the IMM. To reflect the fact that long-chain FA anions cannot bind to UCP1 on the matrix side [19], the access to the substrate binding site in the m-state is shown narrower as compared to c-state. Right panel: an original trace of steady UCP1 current induced by short-chain low-pKa FA analogs. Voltage protocol is shown above. (B) Left panel: the mechanism of transient UCP1 current induced by long-chain low-pKa FA analogs added on the cytosolic face of the IMM. A long-chain FA− analog is translocated by UCP1 similar to short-chain FA−, however the long carbon tail of FA− establishes strong hydrophobic interaction with UCP1 to prevent FA− dissociation. Thus, the negatively charged FA− shuttles within the UCP1 translocation pathway in response to the transmembrane voltage, producing transient currents. These currents suggest that the UCP1 substrate binding site changes its position within the membrane during c-m conformation change. Right panel: an original trace of transient UCP1 current induced by long-chain low-pKa FA analogs. (C) Left panel: the mechanism of H+ current via UCP1 induced by regular long-chain FA added on the cytosolic face of the IMM. UCP1 operates as a symporter that transports one FA− and one H+ per the transport cycle. The H+ and the FA− are translocated by UCP1 upon a conformational change, and H+ is released on the opposite side of the IMM, while the FA− stays associated with UCP1 due to the hydrophobic interactions established by its carbon tail. The FA− anion then returns to initiate another H+ translocation cycle. Charge is translocated only in step 3 when the long chain FA anion returns without the H+. Right panel: an original trace of H+ current via UCP1 induced by regular long-chain FA.