Figure 1.
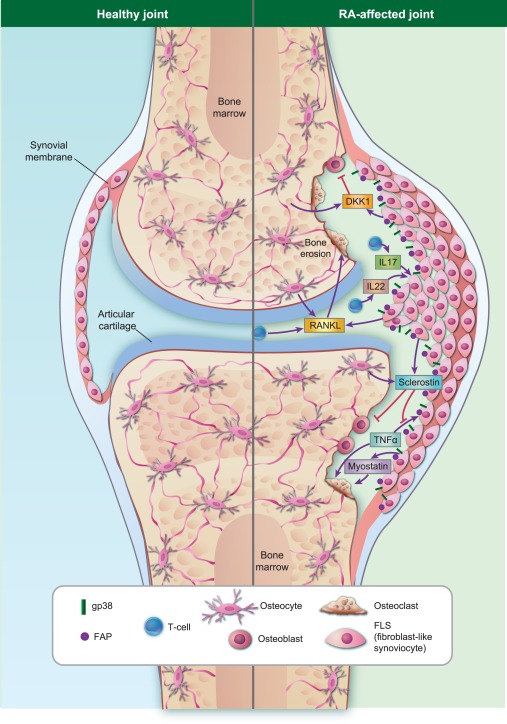
The role of fibroblast‐like synoviocytes (FLS) in inflammatory bone destruction. Under healthy conditions, there is a balance between bone formation and bone destruction to replace old bone tisssue and to repair bone defects. In rheumatoid arthritis (RA), more bone is degraded by osteoclasts than is created by osteoblasts, shifting the balance towards bone destruction. During inflammation, stromal FLS, located in the synovial membrane of the joint space, are able to influence this balance directly or indirectly. FLS release receptor‐activator of nuclear factor‐kappa B (RANKL) in response to inflammatory cytokines such as tumour necrosis factor (TNF)‐α, which subsequently stimulates osteoclastogenesis directly. They also communicate with T cells or release inhibitors of bone formation, such as sclerostin and Dickkopf‐1 (DKK1). In contrast to DKK1, sclerostin not only blocks osteoblast differentiation but also inhibits specifically TNF‐mediated bone destruction, suggesting a protective effect in TNF‐mediated bone loss. Other factors released by FLS, such as myostatin, activates bone destruction directly. Different subsets of FLS, especially gp38+ and FAP+‐expressing FLS, are highly migratory and invasive and seem to be important for cartilage and bone destruction.
