Abstract
During ischemic stroke, neurons and glia are subjected to damage during the acute and neuroinflammatory phases of injury. Production of reactive oxygen species (ROS) from calcium dysregulation in neural cells and the invasion of activated immune cells are responsible for stroke-induced neurodegeneration. Scientists have failed thus far to identify antioxidant-based drugs that can enhance neural cell survival and improve recovery after stroke. However, several groups have demonstrated success in protecting against stroke by increasing expression of antioxidant enzymes in neural cells. These enzymes, which include but are not limited to enzymes in the glutathione peroxidase, catalase, and superoxide dismutase families, degrade ROS that otherwise damage cellular components such as DNA, proteins, and lipids. Several groups have identified cellular therapies including neural stem cells and human umbilical cord blood cells, which exert neuroprotective and oligoprotective effects through the release of pro-survival factors that activate PI3K/Akt signaling to upregulation of antioxidant enzymes. Other studies demonstrate that treatment with soluble factors released by these cells yield similar changes in enzyme expression after stroke. Treatment with the cytokine leukemia inhibitory factor increases the expression of peroxiredoxin IV and metallothionein III in glia and boosts expression of superoxide dismutase 3 in neurons. Through cell-specific upregulation of these enzymes, LIF and other Akt-inducing factors have the potential to protect multiple cell types against damage from ROS during the early and late phases of ischemic damage.
Keywords: Ischemic Stroke, Oxidative Stress, Antioxidant Enzymes, Neuroprotection, Leukemia Inhibitory Factor
1.1 Oxidative Stress in Ischemic Stroke
1.1.1 Production of Reactive Oxygen Species
Oxidative stress is characterized by the excess production of reactive oxygen species (ROS), which may cause irreversible damage to cellular components. Although neural cell damage during stroke is partially triggered by hypoxia, oxidative stress plays an instrumental role during the initial and later phases of ischemic stroke pathophysiology. During the initial phase of injury, energy failure interferes with the activity of ATP-dependent ion channels and the maintenance of the electrochemical gradient (Shenoda, 2015). As a result, neurons experience an increase in excitatory neurotransmission (Khanna et al., 2014). The increase in intracellular Ca2+ triggered by glutamatergic activity activates calmodulin, which is responsible for neuronal nitric oxide synthase activity. Although nitric oxide is not directly neurotoxic, it may react with superoxide anions to form peroxynitrite, an extremely toxic reactive nitrogen species (Dawson et al., 1991). Protein Kinase C, which may be activated by Ca2+ and diacylglycerol, increases activity of NAPDH oxidase, which generates additional ROS (Noh and Koh, 2000).
During the secondary wave of neuroinflammation, ROS are produced by activated microglia/peripheral immune cells. Activated microglia and peripheral macrophages generate nitric oxide via inducible nitric oxide synthase (Merrill et al., 1993). In addition to the release of ROS from activated microglia, these cells also release matrix metalloproteinases that break down the blood-brain barrier (del Zoppo et al., 2007; Shi et al., 2016). Increased blood-brain barrier permeability renders the ischemic hemisphere vulnerable to invading immune cells from the spleen and peripheral immune system (Pennypacker, 2014; Seifert and Pennypacker, 2014). Invading phagocytic cells contribute to oxidative damage in the brain via myeloperoxidase, an enzyme responsible for producing hypochlorous acid, a strong oxidant (Beray-Berthat et al., 2003). NADPH oxidase, which contributes to neural cell damage during the acute phase of stroke pathophysiology, also contributes to ROS production in phagocytic leukocytes such as macrophages and neutrophils (Walder et al., 1997)
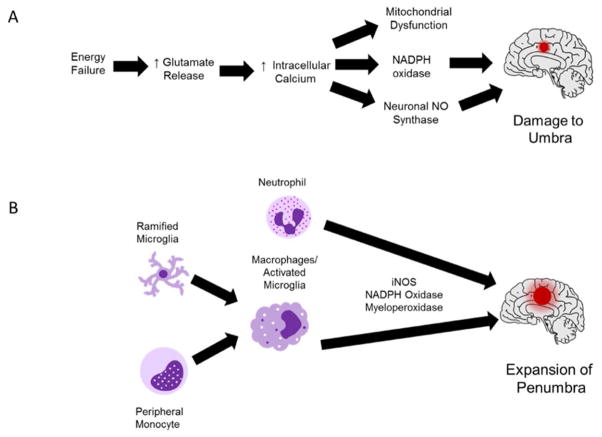
Generation of ROS may cause cellular necrosis by damaging mitochondria and activating pro-apoptotic signaling. Astrocytes facilitate endogenous protection of vulnerable cells, mainly neurons and white matter-forming oligodendrocytes by increasing activity and expression of antioxidant enzymes (Murphy et al., 2001). Since oxidative stress is a key factor behind excitotoxic cell death and neuroinflammation, decreasing ROS remains a focus for research. Some of the mechanisms for ROS-mediated damage during stroke are shown in Figure 1.
Figure 1. ROS-Mediated Damage During the Early and Late Phases of Stroke Pathophysiology.
(A) Acute energy failure is primarily responsible for oxidative damage during the cytotoxic phase of stroke pathophysiology. During the first few minutes to hours after the onset of ischemic stroke, neurons experience a shortage of oxygen and glucose, which interferes with ATP production. Without ATP to maintain the electrochemical gradient, excitotoxic neurotransmission increases and neurons experience an influx of calcium. Enzymes such as NAPDH oxidase and neuronal nitric oxide synthase are activated either directly or indirectly by calcium signaling. High levels of calcium trigger mitochondrial dysfunction, which contributes to ROS production and apoptosis. This phase primarily affects cells in the umbra, which are directly fed by the occluded vessel. (B) From approximately 18 to 96 h after the onset of stroke, the activation of microglia and peripheral leukocytes facilitates neural cell damage. Pro-inflammatory microglia and phagocytic cells (neutrophils/macrophages) produce ROS via enzymes such as inducible nitric oxide synthase, NADPH oxidase, and myeloperoxidase. This “respiratory burst” damages cells adjacent to the ischemic core (the penumbra) and increases the volume of the infarct.
1.1.2 Failure of Exogenous Antioxidants in Stroke Clinical Trials
Despite the role of oxidative stress in neural cell damage during stroke, the performance of exogenous antioxidants in clinical trials has been inconsistent. Some pro-antioxidant drugs have yielded positive results in clinical trials. Ebselen, a glutathione peroxidase (GSH-PX) mimetic that improved outcomes after transient FCI in rodents, was used in a double blind clinical trial for acute ischemic stroke patients. According to the results of the study, patients that received oral Ebselen within 24 hr of stroke onset showed improvement on the Glasgow Outcome Scale at 1 month after injury compared to patients who received the placebo. Unfortunately, there was no significant improvement at 3 months post-stroke (Dawson et al., 1995; Yamaguchi et al., 1998). Clinical trials for “free radical scavengers” have yielded more negative results compared to successful treatments. Arguably the most notable failed trial was for α-(2-disulfophenyl)-N-tertbutylnitrone, also known as NXY-059. NXY-059 is a nitrone compound that prevents the formation of peroxynitrite by mimicking NO. This drug performed extremely well in rodent and primate models of FCI, and produced significant improvements on the Rankin scale compared to placebo during its initial phase III clinical trial. However, NXY-059 produced no significant improvement compared to placebo in an expanded phase III trial.
Several reasons were given as to why NXY-059 failed in the expanded study. For instance, the drug used in the second trial may have been older than the lot used in the first trial. As a result, this older lot may have undergone oxidation prior to administration, rendering the drug less effective. Secondly, NXY-059 induces vasodilation of cerebral vessels, but does not directly promote survival of neural cells. Pharmacokinetic issues, such as the short half-life of the drug and its inability to easily cross the BBB may have also contributed to its lack of efficacy (Shuaib et al., 2007; Strid et al., 2002; Zivin, 2009). In order to confer longer lasting neuroprotection during ischemic stroke, researchers have begun focusing on therapeutics that enhances of the activity the brain’s endogenous antioxidant enzymes. In fact, tPA has been shown to increase oxidative stress that compromises the blood brain barrier and antioxidants have been utilized to block these effects (Lukic-Panin et al., 2010). Therefore, antioxidant enzyme expression has become a novel target for ischemic stroke treatment and to inhibit adverse effects caused by tPA treatment.
1.2 Endogenous Antioxidant Protection
1.2.1 Glutathione Metabolism
Under physiological and pathophysiological conditions, several enzymes are responsible for regulating redox balance in neurons. Some of these enzymes protect neurons from oxidative stress by maintaining adequate expression levels of the antioxidant glutathione. Glutathione, an oligopeptide containing a cysteine residue, acts as a nucleophile by donating electrons to break the disulfide bonds of oxidized proteins. Once reduced, these proteins are unable to react with other cellular components and cause further damage. Oxidized glutathione molecules will form disulfide bonds with each other until they are converted to reduced glutathione by glutathione reductase (Kidd, 1997; Pompella et al., 2003). GSH may also reduce the accumulation of pro-oxidant xenobiotic agents via the glutathione-S-transferase-dependent conjugation (Friling et al., 1990). Although glutathione is low in neurons, astrocytes may protect neurons by synthesizing and secreting high levels of glutathione in vitro. In addition, neuronal glutathione synthesis is largely dependent upon the release of a cysteine-glycine dipeptide from astrocytes (Dringen et al., 1999; Raps et al., 1989).
In addition to its endogenous antioxidant capabilities, glutathione serves as a cofactor for the GSH-PX family of enzymes. GSH-PX enzymes contain the amino acid selenocysteine, which contains selenium in the space normally occupied by sulfur. Although debate has occurred regarding the function of selenocysteine vs. cysteine, studies suggest that the higher reactivity of selenium allows selenoproteins to scavenge peroxides more efficiently (Brigelius-Flohe, 1999). Some members of the GSH-PX family that are expressed in mammalian tissues include classical GSH-PX, gastrointestinal GSH-PX, plasma GSH-PX, and phospholipid hydroperoxide GSH-PX. All GSH-PX enzymes possess the ability to catalyze the breakdown of organic hydroperoxides, but some isoforms such as plasma GSH-PX are able to reduce more complex hydroperoxides. Phospholipid hydroperoxide GSH-PX and classical GSH-PX are highly stable in brain tissue, and preclinical studies show that upregulation of GSH-PX may occur in response to oxidative damage in animal models of neurodegeneration, including Parkinson’s Disease and Alzheimer’s Disease (Aksenov et al., 1998). GSH-PX activity is also neuroprotective in animal models of stroke. In rodent models of transient middle cerebral artery occlusion (MCAO), overexpression of GSH-PX enzymes as well as treatment with a GSH-PX mimetic (Ebselen) aided in protection against oxidative injury (Dawson et al., 1995; Ishibashi et al., 2002; Weisbrot-Lefkowitz et al., 1998).
1.2.2 Catalase
Catalase, a normal component of cellular peroxisomes, is responsible for converting H2O2 into water and molecular oxygen. Catalase is ubiquitously expressed by neurons and glia of the CNS, and is among the most efficient enzymes found in nature (Vainshtein et al., 1981). A study looking at the antioxidant enzymes in human patients with neurodegenerative diseases demonstrated that patients who had experienced an ischemic stroke or had PD showed lower levels of catalase activity in the brain (Rosario de la Torre et al., 1996). As a result, increasing catalase activity has been examined as a potential therapeutic strategy for ischemic stroke. Neurons in the striatum that overexpress catalase are less susceptible to damage following transient MCAO (Gu et al., 2004). Two independent groups demonstrated that injecting catalase conjugated to polyethylene glycol reduces total infarct size as well as BBB dysfunction after transient MCAO (Liu et al., 1989). However, both of these studies showed that conjugated catalase is most efficacious when administered in conjunction with a polyethylene glycol-conjugated form of another antioxidant, superoxide dismutase.
1.2.3 Superoxide Dismutase
Superoxide dismutase (SOD) family enzymes are responsible for converting superoxide (O2−) anions to H2O2 and H2O. Mammalian cells express three isoforms of SOD: SOD1, SOD2, and SOD3. SOD1, alternatively known as copper/zinc-dependent SOD (Cu/Zn-dependent SOD) is a 32 kDa homodimeric enzyme that is ubiquitously expressed and localized in the cytosol. SOD2, or manganese-dependent SOD, is an 88 kDa homotetrameric enzyme that is localized in the mitochondria. Similar to SOD1, SOD2 exhibits a ubiquitous pattern of expression. SOD3, or extracellular SOD, is also dependent upon copper and zinc and contains a signaling peptide sequence for localization to the endoplasmic reticulum and eventual secretion into the extracellular environment. SOD3 exists as a homotetramer of 135 kDa in humans and mice, but it is primarily dimeric in rats (Carlsson et al., 1996; Zelko et al., 2002). In contrast to the ubiquitous expression of SOD1 and SOD2, levels of SOD3 expression vary greatly between organs. Under physiological conditions, SOD3 mRNA levels are highest in the heart, lung, and pancreas, but low in brain tissue (Folz and Crapo, 1994).
Since oxidative stress is a pathological hallmark of neurodegeneration, SOD dysfunction is implicated in diseases characterized by neuronal loss. Mutations in SOD1 contribute to the development of familial amyotrophic lateral sclerosis (ALS), and multiple studies suggest that reduced SOD2 activity may be associated with Alzheimer’s and Parkinson’s diseases (Azari et al., 2001; Belluzzi et al., 2012; Wiener et al., 2007). However, preclinical studies demonstrate that increased activity of all three SOD enzymes may reduce brain damage after FCI. As previously mentioned, treatment with polyethylene glycol-conjugated catalase and SOD effectively reduced ischemic brain damage. EUK-134, a selenium -manganese complex that acts as a mimetic for SOD and catalase, decreased brain damage when administered 3 h after stroke, a comparable time point to administration of tPA (Baker et al., 1998). Since H2O2 is a product of the reduction of O2−, combination therapy with both enzymes would allow for scavenging of both ROS and better protection against ischemic damage.
Targeting SOD enzyme expression has also shown considerable success in animal models of stroke. Davis et al. showed that delivery of the SOD1 gene using herpes simplex viral vectors protected mice against striatal damage induced by transient MCAO compared to the vector alone (Davis et al., 2007). Unfortunately, the neuroprotective effect of SOD1 overexpression appears to be limited to models of cerebral ischemia/reperfusion injury, since SOD1 overexpressing mice did not show a significant decrease in infarct volume compared to wild-type mice after permanent MCAO. The authors of the latter study suggest that the protective effect of SOD1 overexpression is minimal due to high basal levels of SOD1 in the brain tissue of wild-type mice (Chan et al., 1993). Several independent groups reported that SOD2 knockout mice show greater susceptibility to oxidative stress after permanent and transient MCAO (Kim et al., 2002; Mehta et al., 2011; Mikawa et al., 1995; Murakami et al., 1998). Increasing SOD3 is also an effective strategy in protecting against oxidative damage in models of ischemic stroke. Sheng et al. demonstrated that mice that overexpress the SOD3 gene have smaller infarct volumes compared to wild-type mice (Sheng et al., 1999).
1.3 Factors Affecting Antioxidant Enzyme Expression
1.3.1 Sex-specific Antioxidant Expression
Preclinical and clinical studies indicate that high levels of estrogen protect younger females against the risk of ischemic stroke. In addition to decreasing inflammation, estrogen increases the expression and activity of several antioxidant enzymes in the brain. In a study by Borras et al., female rats were less prone to mitochondrial damage compared to male rats due to higher basal expression of GSH-PX and SOD2 (Borrás et al., 2003). Strehlow et al. showed that 17β-estradiol administration upregulated expression of SOD2 and SOD3 (Strehlow et al., 2003). Reduction of estrogen via ovariectomy reduced expression of these enzymes. In another independent study, removal of the ovaries increased levels of lipid peroxidation in the brain (Özgönül et al., 2003). Among aged females, the incidence of stroke is comparable to that of older males due to lower estrogen production after menopause (Koellhoffer and McCullough, 2013). However, other evidence demonstrates that aged females still have a higher antioxidant capacity compared to aged males. Aged female rats had higher numbers of differentiated mitochondria and uncoupling proteins, which protect mitochondria against ROS (Guevara et al., 2009).
1.3.2 Antioxidant Enzymes Compensate for Increased ROS Production in Aged Brains
According to a study by Driver et al., basal production of ROS in brain tissue increases with age. Using the ROS-sensing dye 2,7-dichlorodihydrofluorescin, the authors showed that there is a significant elevation in ROS production between 21-day old rat brains and adult (3–6 months) rat brains. Among aged rat brains (24 months), there is a significant elevation in ROS production compared to the brains of adult rats (Driver et al., 2000). Higher rates of ROS production in aged brains may be attributed to increased mitochondrial dysfunction that occurs with age (Petrosillo et al., 2008).
Several groups have reported conflicting results regarding the age-related change in brain antioxidant activity. Benzi et al. reported that activities of GSH-PX, glutathione reductase, and SOD decrease in the parieto-temporal cortex, striatum, substantia nigra, and thalamus in rats aged 15–30 months of age compared to younger rats (Benzi et al., 1988).
Other studies show that antioxidant activity is higher in aged brains to compensate for increased ROS production. According to Hussain et al., antioxidant enzyme activity increase with age to compensate for excess generation of ROS. In this particular study, the authors demonstrated that SOD and GSH-PX activity increased in the brain of 2 year old mice compared to young mice (Hussain et al. 1995). In addition to the aging alone, antioxidant activity may be increased by age-related conditions such as Alzheimer’s disease. Lovell et al. demonstrated that higher levels of lipid peroxidation in the brains of Alzheimer’s patients resulted in higher levels of catalase, SOD, and GSH-PX/glutathione reductase activity (Lovell et al., 1995).
Despite the overall increase in brain antioxidant activity with age, studies show that the magnitude of increased enzyme activity varies between sexes. For instance, aged male rats (28–30 months) had significantly elevated SOD activity in several regions of the brain, including the substantia nigra, hippocampus, striatum, and certain regions of the cerebral cortex; compared to young rats (7 months). Although SOD1 activity was significantly higher in the parieto-temporal and occipital regions of the cortex in aged males compared to young males, upregulation of SOD2 activity was primarily responsible for higher total SOD activity in the brains of aged males. Aged female rats showed significant increases in SOD activity in the substantia nigra and cortical regions compared to young females as well as higher SOD2 activity in the hippocampus and occipital cortex. However, elevations in SOD activity were less pronounced in aged females compared to aged males, and SOD1 activity remained unchanged in female rats. Aged female rats had significantly higher catalase activity in the cerebellum, whereas aged male rats showed no significant changes in brain catalase activity compared to their younger counterparts (Carrillo et al., 1992). Sobocanec at al. verified that catalase activity increases with age in female mice, but not in males. Moreover, this study showed that females experience higher levels of GSH-PX activity at 18 months of age compared to 18 month males. These data demonstrate that while aging favors an increased antioxidant profile in the brain, the activities of individual enzymes vary between the sexes (Sobočanec et al., 2003).
1.4 Therapeutic Strategies that Upregulate Antioxidant Enzymes
1.4.1 Human Umbilical Cord Blood Cells
Although these therapies contain mesenchymal stem cells, cells isolated from autologous peripheral and umbilical cord blood also contain a large fraction of mononuclear cells, which consist of monocytes/macrophages, lymphocytes, and neutrophils. Similar to NSCs, these cell populations have been shown to exert protective effects via released factors that activate survival signaling. HUCB treatment 48 h after stroke increases PI3K/Akt signaling and induces the expression of antioxidant enzymes. The cerebroprotective potential of cellular therapies lies in their ability to turn on pro-survival signaling in neural cells via paracrine mechanisms. Through the activation of Akt, these factors increase the activity of TFs such as MZF-1 and Sp1 and promote increased expression of protective genes. Among oligodendrocytes, several of these protective genes included enzymes that reduce the production of ROS during in vivo and in vitro ischemia. HUCB treatment increased the expression of peroxiredoxin IV (Prdx4) and metallothionein III (Mt3), two antioxidant enzymes (Rowe et al., 2010; Rowe et al., 2012).
1.4.2 Neural Stem Cell Therapy
Among preclinical rodent studies, neural stem cells (NSCs) contributed to brain repair through several mechanisms. These cells, which are derived from neurogenesis sites in the subventricular and subgranular zones, facilitate tissue repair by proliferating and differentiating into neurons and glia (Zhang et al., 2004). Preclinical studies indicate that NSCs confer neuroprotection through the release of neurotropic and anti-inflammatory factors as well. NSC lines may be readily manipulated to release higher quantities of neuroprotective factors. Various factors released by NSCs have been shown to promote neural repair in models of SCI (Lu et al., 2003), EAE (Laterza et al., 2013), and ICH (Lee et al., 2007). According to Martinez-Serrano, grafted NSCs that produced higher levels of BDNF and NGF protect GABAergic striatal neurons from excitotoxic death, thus giving these cells promise as a treatment for HD (Martínez-Serrano and Björklund, 1996). Since NGF and BDNF are potent inducers of Akt signaling, NSCs that overexpress these two factors could protect other types of neurons against cytotoxicity through Akt-dependent gene expression (Nguyen et al., 2010).
Despite the neurotrophic actions of factors released from NSCs, the ability of these cells to upregulate antioxidant genes has not been widely explored. However, there is evidence that certain NSC populations induce expression of SOD2. Madhaven et al. demonstrated that NSCs produced high levels of CNTF, VEGF, and BDNF following stimulation with 3-nitropropionic acid, a mitochondrial toxin that has been used to induce degeneration of striatal neurons (Beal et al., 1993; Madhavan et al., 2008). These neurotrophic factors and cytokines significantly upregulated SOD2 expression in neurons, which reduced subsequent 3-nitropropionic acid-induced death in vitro. Other studies demonstrate some of the factors produced by NSCs can reproduce the protective effects of cellular treatment while avoiding the inconsistencies associated with these types of therapies. One such factor that confers protective gene expression in neural cells is leukemia inhibitory factor (LIF) (Chen et al., 2010).
1.4.3 Leukemia Inhibitory Factor
LIF is a glycoprotein in the IL-6 family of cytokines that inhibits self-renewal of murine leukemia cell lines and promotes self-renewal of embryonic stem cells. This particular study identified several other cell types that participate in LIF signaling including osteoblasts, adipocytes, hepatic parenchymal cells, and neurons (Tomida et al., 1984). LIF exerts its signaling through LIF receptor/glycoprotein 130 (LIFR/gp130) complex and activates the following downstream signaling cascades: Raf/MEK/ERK (Arthan et al., 2010), JAK/STAT (Stahl et al., 1994), and PI3K/Akt (Oh et al., 1998). Similar to other cytokines, LIF has a short terminal half-life of approximately 2 h (Segrave et al., 2004). Therefore, the long-term effects of LIF signaling primarily occur through changes in genes that regulate differentiation, survival, and self-renewal (Graf et al., 2011).
In addition to controlling stem cell fate, the effects of LIF extend to neural cell development in the CNS/PNS. Several groups reported that LIF is identical to a myocardium-derived protein that promotes the survival and development of cholinergic motor neurons in vivo and in vitro (Curtis et al., 1994; Martinou et al., 1992; Yamamori et al., 1989). NSC populations also require LIF for self-renewal. In a study performed by Bauer and Patterson, adenoviral vectors were used to overexpress LIF in the brain. Mice that received the adenoviral-LIF construct showed lower rates of neurogenesis in the subventricular zone compared to mice that received the adenoviral-LacZ construct. LIF overexpression also increased numbers of cells that stained positive for bromodeoxyuridine (BrdU) and the glial cell markers S100 and Oligo2, while decreasing the number of cells staining positive for DCX, a marker of immature neurons. These results demonstrate that LIF inhibits neurogenesis in the subventricular zone, but helps increase the brain’s capacity to repair it by maintaining high populations of NSCs and glial progenitor cells. (Bauer and Patterson, 2006). By contrast, CNTF, another IL-6 family cytokine that depends on the LIFR subunit for signaling, promotes neurogenesis in the forebrain and hypothalamus (Emsley and Hagg, 2003; Kokoeva et al., 2005).
1.5 Antioxidant Enzymes upregulated by LIF
Since LIF is among the factors produced by HUCB cell (Seo et al., 2011), LIF does promote antioxidant expression and reduce white matter damage after permanent FCI in a manner similar to HUCB therapy. Rats were administered either LIF or PBS at 6, 24, and 48 h after undergoing permanent MCAO and euthanized at 72 h. According to the results of this study, LIF treatment increased motor skill recovery and reduced stroke volume at 72 h post-MCAO. White matter showed considerably lower levels of damage in vivo and significantly lower levels of cytotoxicity following in vitro ischemia. Not only were the in vitro protective effects of LIF (200 ng/mL) dependent upon Akt signaling, but mRNA levels of the antioxidant genes peroxiredoxin IV (Prdx4) and metallothionein III (Mt3) were significantly higher among oligodendrocytes that received LIF prior to OGD compared to those that received PBS (Rowe, 2011; Rowe et al., 2014).
1.5.1 Peroxiredoxin IV
Peroxiredoxin (Prdx) family enzymes, or “thiol-specific antioxidants,” were initially identified due to their ability to reduce oxidized proteins in the presence of dithiothreitol, which reduced molecular O2 to form several ROS species (Chae et al., 1993). Similar to GSH-PX, Prdx enzymes are able to form disulfide linkages following oxidation, and return to their active form following reduction by an additional enzyme, thioredoxin (Rhee et al., 2001). During each redox cycle, Prdx enzymes catabolize the conversion of H2O2 into H2O and O2. However, a study by Bryk et al. indicated that Prdx isoforms in bacteria have the capacity to scavenge peroxynitrite (ONOO−) (Bryk et al., 2000). Six Prdx isoforms are expressed in mammals; the cytosolic isoforms Prdx1, Prdx2, and Prdx6; the mitochondrial isoform Prdx3; the secreted isoform Prdx4; and Prdx5, which is localized in multiple organelles (Rhee et al., 2012). In the brain, neurons express Prdx2-5 while Prdx1, 4, and 6 are localized to glial cells (Goemaere and Knoops, 2012). Currently, the role of Prdx enzymes in neural cell protection remains controversial. Studies have identified pro-survival mechanism for Prdx 2, 4, and 5 during in vivo and in vitro ischemia. Shichita et al. demonstrated that antibodies against Prdx 1, 2, 5, and 6 decreased infarct volume and decreased the release of IL-23 from macrophages after transient MCAO (Shichita et al., 2012). This paradoxical role for Prdx enzymes in stroke pathology may be explained by several factors. The protective role for Prdx4-5 during stroke was demonstrated using rodent models of permanent MCAO, suggesting that differences between permanent and transient FCI pathology may determine whether Prdx enzymes contribute to neuroprotection vs. neuroinflammation (Rowe et al., 2010; Shahaduzzaman et al., 2015). In addition, the time point at which the anti-Prdx antibodies are administered may determine whether or not blocking their activity is neuroprotective. The studies showing the neuroprotective role of Prdx2 were performed in vitro, meaning that this isoform may only be neuroprotective in the absence of invading leukocytes (Boulos et al., 2007; Rowe et al., 2010; Rowe et al., 2012; Rowe et al., 2014; Shichita et al., 2012; Vendrame et al., 2005).
Under resting conditions, Prdx4 is localized to the endoplasmic reticulum. Within the ER, Prdx4 is involved with protein disulfide isomerase (PDI)-mediated protein folding. When oxidized by hydrogen peroxide, Prdx4 forms a homodimer via disulfide linkages. This Prdx4 homodimer is reduced by PDI, which transfers the disulfide linkage to proteins undergoing folding in the ER [88]. In addition to its role in the endoplasmic reticulum, several pathophysiological conditions can trigger the upregulation and release of Prdx4. Some of these conditions include excess production of ROS/RNS, inflammatory mediators, and stimuli that trigger apoptosis. Furthermore, Rowe et al. determined that Prdx4 is upregulated specifically through Akt signaling.
During disease states, Prdx4 is secreted into the extracellular environment to scavenge peroxides. Okado-Matsumoto et al. demonstrated that Prdx4 easily binds to heparin in its reduced form [96]. This characteristic of Prdx4 allows it to reduce tissue damage in a manner similar to other extracellular enzymes such as plasma GSH-PX and SOD3 (Okado-Matsumoto et al., 2000). Prdx4 can also exist in a decameric form, which allows it to be transported in the systemic circulation (Cao et al., 2011). However, studies suggest that increased expression of intracellular or membrane-bound Prdx4 are preferable to high expression levels of Prx4 in the serum. For instance, Nickel et al. showed that emergency room patients who had increased expression of serum Prdx4 were more likely to present symptoms of severe disease compared to patients with lower expression (Nickel et al., 2011). One explanation for this phenomenon is that higher expression of Prdx4 in the serum correlates with lower cellular levels. Lower expression of Prdx4 in the cell could lead to compromised antioxidant protection, even with the presence of other Prdx isoforms in the cell (Nickel et al., 2011).
Prdx4 is induced by LIF and HUCB therapy. Considering the oligoprotective effects of LIF and upregulation of Prdx4 were blocked by inhibitors of Akt, it is likely that other Akt-activating drugs may upregulate Prdx4 as well (Leonardo et al., 2012; Rowe et al., 2010; Rowe et al., 2014).
1.5.2 Metallothionein III
Metallothionein III (Mt3) is another antioxidant enzyme induced by HUCB cell treatment and LIF during FCI. Mt3 is a member of the Zn2+-containing metallothionein family that is expressed exclusively in neural tissue (Masters et al., 1994). According to Lee et al., Mt3 regulates several processes including Zn2+ sequestration, inhibition of neurite formation, and autophagy. Due to its ability to inhibit neurite outgrowth in the presence of brain extracts from AD patients, it is alternatively called growth inhibitory factor (GIF). However, Mt3 is not able to perform this function in the absence of these extracts (Lee et al., 2003). Although Mt1 and Mt2 are upregulated in AD, Mt3 is downregulated in the brain of AD patients (Yu et al., 2001). High concentrations of ions such as Cu2+ and Zn2+ are present at synapses in the AD brain. Cu2+ ions bind to and facilitate Aβ aggregation. However, Meloni et al. showed that Mt3 reduces Cu2+ found in these aggregates and reduces amyloid beta (Aβ)-mediated cytotoxicity and ROS production (Meloni et al., 2008). Therefore, lower expression of Mt3 exacerbate Aβ plaque formation in the AD brain.
In addition to its ability to prevent plaque formation, Mt3 also directly protects against superoxide, hydroxyl radicals, and hydrogen peroxide (Thornalley and Vašák, 1985). You et al. demonstrated that Mt3 protected neurons in vitro against ROS-mediated DNA strand breaks and deoxyribose degradation (You et al., 2002). Since DNA damage from ROS generation is a key cause of death during the excitotoxic phase of ischemic stroke, increased Mt3 in the white matter would protect oligodendrocytes against apoptosis (Li et al., 2011). Other studies show that Mt3 also scavenges ROS in the extracellular environment. Although Mt3 does not contain a signal peptide, El Ghazi et al. discovered that astrocytes secrete Mt3 via a multi-protein complex (El Ghazi et al., 2006).
1.5.3 Superoxide Dismutase 3
LIF protects neurons during permanent FCI in addition to oligodendrocytes via upregulation of antioxidant enzymes. Rats that were administered LIF after MCAO showed significantly elevated levels of total SOD activity in tissue from the ipsilateral hemisphere compared to rats that received PBS. This increase in SOD activity did not correspond to upregulation of SOD1 or SOD3, but rats with elevated SOD activity in the brain showed significantly higher levels of SOD3 expression in brain tissue and neurons of the ipsilateral cerebral cortex. LIF (200 ng/mL) significantly reduced death in an Akt-dependent manner and increased SOD3 mRNA after OGD among primary cortical neurons. Transfection with siRNA against SOD3 prior to treatment with LIF counteracted the decrease in LDH release and caspase-3 cleavage seen with LIF treatment alone (Davis et al., 2016).
The effectiveness of SOD3 overexpression as neuroprotective strategy stems from its low levels of basal expression in the brain. While SOD1 and SOD2 are highly expressed under basal conditions, SOD3 is only induced in neurons during injury (Fukui et al., 2002). However, SOD3-overexpressing mice show significantly lower levels of brain damage after transient stroke compared to wild-type mice (Sheng et al., 1999). The difference in antioxidant capacity between SOD3 transgenic mice and wild-type mice might be greater than the difference between SOD1/SOD2 transgenic mice and wild-type mice. These data and studies by other groups demonstrate that SOD3 upregulation may confer potent neuroprotective effects during permanent FCI. In addition, several studies have shown that SOD3 gene expression is regulated by MZF-1 and Sp1, two TFs implicated in the protective effects of HUCB treatment (Zelko and Folz, 2003; Zelko and Folz, 2004).
SOD3, similar to the LIF-inducible enzyme Prdx4, protects tissue against damage from ROS when it is secreted into the extracellular environment. SOD3 contains a signaling peptide that allows for its secretion into the extracellular space (Zelko et al., 2002). SOD3 also contains a heparin-binding domain that allows it to attach to the extracellular matrix (Antonyuk et al., 2009). Prdx enzymes catalyze the breakdown of hydrogen peroxide to water and oxygen, but cannot break down superoxide. Even with increased Prdx4 expression, oligodendrocytes are still vulnerable to superoxide-mediated damage during cerebral ischemia. Likewise, SOD3 breaks down superoxide but is unable to process hydrogen peroxide. Prdx4 and Mt3 prevent buildup of hydrogen peroxide that is produced as a product of the SOD reaction.
Since superoxide reacts with free NO to form peroxynitrite, SOD3 indirectly increases NO bioavailability in the cerebral vasculature. NO promotes tissue repair via several mechanisms. NO production activates protein kinase G by increasing cGMP synthesis by guanylate cyclase. PKG increases cerebral blood flow in the ischemic hemisphere by promoting vasodilation of large cerebral vessels (Moncada and Higgs 1993). In the cerebral microvasculature, endothelial NO synthase activity increases synthesis of vascular endothelial growth factor, which promotes angiogenesis (Lee et al., 1999). The synthesis of new blood vessels improves perfusion of the infarct by diverting blood flow around the occluded vessel. In addition to its effects on vascular tone and angiogenesis, NO also promotes synthesis of brain-derived neurotrophic factor (BDNF), a protective growth factor. In a study by Chen et al., endothelial NO synthase knockout mice had larger infarct volumes at 24 h post-MCAO, decreased neurogenesis in the subventricular zone, and impaired angiogenesis compared to their wild-type counterparts (Chen et al., 2005).
The increased SOD activity and SOD3 expression in neurons observed after LIF treatment differ from the reduction in SOD activity observed in oligodendrocyte after LIF treatment. Blocking Prdx4 not only increased LDH release, but it returned SOD activity to levels similar to the cells treated with PBS prior to OGD. The decrease in SOD activity coincides with increased antioxidant protection from Prdx4, but inhibiting Prdx4 did not significantly increase SOD activity compared to the OGD alone treatment group. While oligodendrocytes increase SOD activity in response to oxidative stress, they aren’t able to increase activity to compensate for decreased Prdx4. By contrast, neurons treated with LIF had significantly higher levels of SOD3 siRNA compared to neurons treated with PBS. Cells transfected with SOD3 siRNA and treated with LIF showed no significant decrease in cell death compared to neurons transfected with scrambled siRNA and treated with PBS. Therefore, the upregulation of SOD3 after LIF treatment is a phenomenon that is unique to neurons, but not observed in oligodendrocytes. While Increased Prdx4 and Mt3 expression are a hallmark of LIF-mediated oligoprotection, SOD3 upregulation is a neuron-specific mechanism of protection (Davis et al., 2016; Rowe, 2011; Rowe et al., 2014).
1.6 Antioxidant Effects of Other IL-6 Family Cytokines
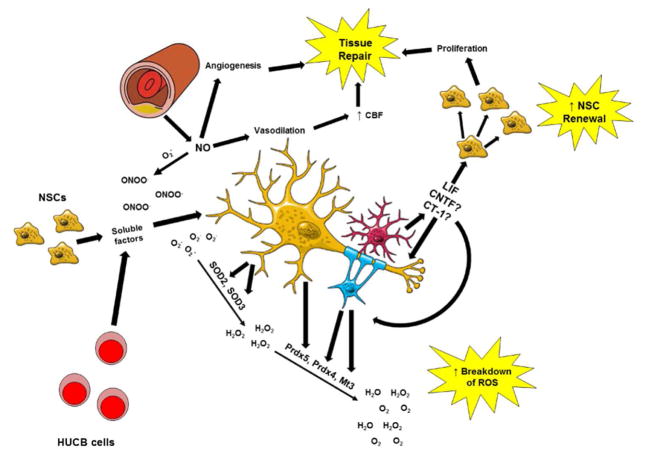
Despite the failures of exogenous agents, focusing research efforts on drugs that confer long-lasting changes in antioxidant expression have shown great potential in treating animal models of stroke. Anti-inflammatory cytokines provide a unique benefit to stroke patients: they exert their signaling quickly before being cleared from the body, thus lessening the potential for adverse side effects. However, the downstream effects of these signaling pathways confer long-lasting protection by increasing the antioxidant capacity of neural cells. Rowe et al. demonstrated that Akt-inducible antioxidant genes are regulated by several common TFs (Rowe et al., 2010). Identifying drugs that increase the activity of Akt and these TFs could provide multifaceted protection against ischemic oxidative damage (Figure 2).
Figure 2. The Synergistic Effects of Pro-Antioxidant Therapeutics.
Cellular therapies, including HUCB cells and NSCs, exert their protective actions via the release of soluble factors which activate pro-survival signaling cascades. Through the release of these factors, NSCs are able to increase expression of SOD2 while HUCB cells are able to increase expression of Prdx4/Mt3 in oligodendrocytes and Prdx5 in neurons. LIF, and possibly other IL-6 family cytokines, exerts similar effects on pro-survival signaling and increases expression of SOD3 in neurons and Prdx4/Mt3 in oligodendrocytes. In addition to the direct effect on antioxidant expression, LIF reduces oxidative stress during ischemic stroke by promoting the self-renewal of NSCs and preventing the formation of peroxynitrite (ONOO-) by reacting with superoxide. By decreasing the formation of peroxynitrite, nitric oxide increases angiogenesis and promotes vasodilation in the infarct. Both of these processes increase perfusion of ischemic tissue and contribute to brain repair.
Several therapeutic agents reduce neural cell damage through induction of antioxidant enzymes. In addition to LIF, other cytokines in the IL-6 family activate similar signaling pathways. For instance, Alonzi et al. demonstrated that LIF and CNTF promote the survival of sensory neurons in the nodose ganglion through PI3K/Akt and STAT3 signaling (Alonzi et al., 2001). Cardiotrophin-1 (CT-1), another cytokine in the IL-6 family, protects cultured neurons against pro-oxidant agents including ferrous sulfate and 3-morpholinosydnonimine (SIN-1). This neuroprotective effect of CT-1 mirrors its protective effects on cardiomyocytes during ischemia/reperfusion injury, which occur through simultaneous activation of PI3K/Akt, MAPK, and JAK/STAT signaling. Although the authors of this study did not measure quantitative changes in the expression of antioxidant genes, the activation of Akt-dependent transcription factors by CNTF contributes to protection against SIN-1 and ferrous sulfate (Wen et al., 2005). One common factor shared by LIF, CNTF, and Cardiotrophin-1, in addition to being in the IL-6 family of cytokines, is that they require LIFR for downstream signaling. According to Arce et al., LIF, CNTF, and CT-1 increase the survival of motor neurons derived from wild-type mouse embryos. However, derived from homozygous Lifrβ−/− knockout mice are immune to the protective signaling of these cytokines. These data provide a case for enhancing endogenous antioxidant protection through LIFR-dependent factors (Arce et al., 1999).
1.7 Conclusion
One of the current shortcomings of clinical stroke treatments stems from the inability to address both phases of stroke pathophysiology. Increased expression of enzymes such as Prdx4, Mt3, and SOD3 would not only protect neural cells against ROS that are generated during the acute cytotoxic phase of damage, but also against ROS produced by activated microglia and peripheral leukocytes. Moreover, these antioxidant enzymes can be modified (polyethylene glycol) to increase their stability thus allowing exogenous application as a stroke treatment. In addition, several of the therapies discussed in this review have larger therapeutic windows compared to intravenous tPA therapy, which would allow them to efficiently protect the brain in stroke patients that could not receive thrombolytic therapy within its therapeutic window. These promising results pre-clinical studies will hopefully open the door for clinical trials involving agents that increase antioxidant expression. By increasing the antioxidant capacity of the brain during stroke, these drugs have the potential to reduce brain damage and mortality among patients while improving recovery of stroke survivors.
Highlights.
Overview of antioxidants as treatment for stroke
Explanation of failed attempts to develop exogenous antioxidant treatment
Overview of endogenous antioxidant systems
Use of agents that activate these endogenous antioxidant systems to develop stroke therapeutic
Acknowledgments
Funding
This study was funded by project #7R01NS091146-02 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Stephanie M. Davis declares that she has no conflict of interest. Keith R. Pennypacker declares that he has no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksenov MY, Tucker HM, Nair P, Aksenova MV, Butterfield DA, Estus S, Markesbery WR. The expression of key oxidative stress-handling genes in different brain regions in Alzheimer’s disease. J Mol Neurosci. 1998;11:151–164. doi: 10.1385/JMN:11:2:151. [DOI] [PubMed] [Google Scholar]
- Alonzi T, Middleton G, Wyatt S, Buchman V, Betz AKU, Muller W, Musiani P, Poli V, Davies AM. Role of STAT3 and PI 3-Kinase/Akt in Mediating the Survival Actions of Cytokines on Sensory Neurons. Molecular and Cellular Neuroscience. 2001;18:270–282. doi: 10.1006/mcne.2001.1018. [DOI] [PubMed] [Google Scholar]
- Antonyuk SV, Strange RW, Marklund SL, Hasnain SS. The structure of human extracellular copper–zinc superoxide dismutase at 1.7 Å resolution: insights into heparin and collagen binding. J Mol Bio. 2009;388:310–326. doi: 10.1016/j.jmb.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Arce V, Garces A, de Bovis B, Filippi P, Henderson C, Pettmann B, deLapeyrière O. Cardiotrophin-1 requires LIFRβ to promote survival of mouse motoneurons purified by a novel technique. J Neurosci Res. 1999;55:119–126. doi: 10.1002/(SICI)1097-4547(19990101)55:1<119::AID-JNR13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Arthan D, Hong SK, Park JI. Leukemia inhibitory factor can mediate Ras/Raf/MEK/ERK-induced growth inhibitory signaling in medullary thyroid cancer cells. Cancer Letters. 2010;297:31–41. doi: 10.1016/j.canlet.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Azari MF, Galle A, Lopes EC, Kurek J, Cheema SS. Leukemia inhibitory factor by systemic administration rescues spinal motor neurons in the SOD1 G93A murine model of familial amyotrophic lateral sclerosis. Brain Res. 2001;922:144–147. doi: 10.1016/s0006-8993(01)03156-0. [DOI] [PubMed] [Google Scholar]
- Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic Combined Superoxide Dismutase/Catalase Mimetics Are Protective as a Delayed Treatment in a Rat Stroke Model: A Key Role for Reactive Oxygen Species in Ischemic Brain Injury. J Pharm Exp Therap. 1998;284:215–221. [PubMed] [Google Scholar]
- Bauer S, Patterson PH. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci. 2006;26:12089–12099. doi: 10.1523/JNEUROSCI.3047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13:4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi E, Bisaglia M, Lazzarini E, Tabares L, Beltramini M, Bubacco L. Human SOD2 modification by dopamine quinones affects enzymatic activity by promoting its aggregation: possible implications for Parkinson’s disease. PLoS ONE. 2012;7:e38026. doi: 10.1371/journal.pone.0038026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzi G, Pastoris O, Villa RF. Changes induced by aging and drug treatment on cerebral enzymatic antioxidant system. Neurochem Res. 1988;13:467–478. doi: 10.1007/BF01268883. [DOI] [PubMed] [Google Scholar]
- Beray-Berthat V, Croci N, Plotkine M, Margaill I. Polymorphonuclear neutrophils contribute to infarction and oxidative stress in the cortex but not in the striatum after ischemia–reperfusion in rats. Brain Res. 2003;987:32–38. doi: 10.1016/s0006-8993(03)03224-4. [DOI] [PubMed] [Google Scholar]
- Borrás C, Sastre J, García-Sala D, Lloret A, Pallardó FV, Viña J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radical Biology and Medicine. 2003;34:546–552. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- Boulos S, Meloni BP, Arthur PG, Bojarski C, Knuckey NW. Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J Neurosci Res. 2007;85:3089–3097. doi: 10.1002/jnr.21429. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- Cao Z, Tavender TJ, Roszak AW, Cogdell RJ, Bulleid NJ. Crystal structure of reduced and of oxidized peroxiredoxin IV enzyme reveals a stable oxidized decamer and a non-disulfide-bonded intermediate in the catalytic cycle. J Biol Chem. 2011;286:42257–42266. doi: 10.1074/jbc.M111.298810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L, Marklund S, Edlund T. The rat extracellular superoxide dismutase dimer is converted to a tetramer by the exchange of a single amino acid. Proc Natl Acad Sci. 1996;93:5219–5222. doi: 10.1073/pnas.93.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo MC, Kanai S, Sato Y, Kitani K. Age-related changes in antioxidant enzyme activities are region and organ, as well as sex, selective in the rat. Mechanisms of ageing and development. 1992;65:187–198. doi: 10.1016/0047-6374(92)90035-c. [DOI] [PubMed] [Google Scholar]
- Chae HZ, Kim IH, Kim K, Rhee SG. Cloning, sequencing, and mutation of thiol-specific antioxidant gene of Saccharomyces cerevisiae. J Bio Chem. 1993;268:16815–16821. [PubMed] [Google Scholar]
- Chan PH, Kamii H, Yang G, Gafni J, Epstein CJ, Carlson E, Reola L. Brain infarction is not reduced in SOD-1 transgenic mice after a permanent focal cerebral ischemia. NeuroReport. 1993;5:293–296. doi: 10.1097/00001756-199312000-00028. [DOI] [PubMed] [Google Scholar]
- Chen HC, Ma HI, Sytwu HK, Wang HW, Chen CCV, Liu SC, Chen CH, Chen HK, Wang CH. Neural stem cells secrete factors that promote auditory cell proliferation via a leukemia inhibitory factor signaling pathway. J Neurosci Res. 2010;88:3308–3318. doi: 10.1002/jnr.22492. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, Lindsay RM, DiStefano PS. Retrograde axonal transport of LIF is increased by peripheral nerve injury: correlation with increased LIF expression in distal nerve. Neuron. 1994;12:191–204. doi: 10.1016/0896-6273(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Davis AS, Zhao H, Sun GH, Sapolsky RM, Steinberg GK. Gene therapy using SOD1 protects striatal neurons from experimental stroke. Neuroscience Letters. 2007;411:32–36. doi: 10.1016/j.neulet.2006.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Collier LA, Leonardo CC, Seifert HA, Ajmo CT, Jr, Pennypacker KR. Leukemia Inhibitory Factor Protects Neurons from Ischemic Damage via Upregulation of Superoxide Dismutase 3. Mol Neurobiol. 2016 doi: 10.1007/s12035-015-9587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D, Masayasu H, Graham D, Macrae I. The neuroprotective efficacy of ebselen (a glutathione peroxidase mimic) on brain damage induced by transient focal cerebral ischaemia in the rat. Neurosci Letters. 1995;185:65–69. doi: 10.1016/0304-3940(94)11226-9. [DOI] [PubMed] [Google Scholar]
- Dawson V, Dawson T, London E, Bredt D, Snyder S. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. Microglial Activation and Matrix Protease Generation During Focal Cerebral Ischemia. Stroke. 2007;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the Antioxidant Glutathione in Neurons: Supply by Astrocytes of CysGly as Precursor for Neuronal Glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver AS, Kodavanti PRS, Mundy WR. Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxic Teratology. 2000;22:175–181. doi: 10.1016/s0892-0362(99)00069-0. [DOI] [PubMed] [Google Scholar]
- El Ghazi I, Martin B, Armitage I. Metallothionein-3 is a component of a multiprotein complex in the mouse brain. Exp Bio Medicine. 2006;231:1500–1506. doi: 10.1177/153537020623100908. [DOI] [PubMed] [Google Scholar]
- Emsley J, Hagg T. Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice. Exp Neuro. 2003;183:298–310. doi: 10.1016/s0014-4886(03)00129-8. [DOI] [PubMed] [Google Scholar]
- Folz RJ, Crapo JD. Extracellular Superoxide Dismutase (SOD3): Tissue-Specific Expression, Genomic Characterization, and Computer-Assisted Sequence Analysis of the Human EC SOD Gene. Genomics. 1994;22:162–171. doi: 10.1006/geno.1994.1357. [DOI] [PubMed] [Google Scholar]
- Friling R, Bensimon A, Tichauer Y, Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci U S A. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Ookawara T, Nawashiro H, Suzuki K, Shima K. Post-ischemic transcriptional and translational responses of EC-SOD in mouse brain and serum. Free Radic Biol Med. 2002;32:289–298. doi: 10.1016/s0891-5849(01)00804-8. [DOI] [PubMed] [Google Scholar]
- Goemaere J, Knoops B. Peroxiredoxin distribution in the mouse brain with emphasis on neuronal populations affected in neurodegenerative disorders. J Comp Neurol. 2012;520:258–280. doi: 10.1002/cne.22689. [DOI] [PubMed] [Google Scholar]
- Graf U, Casanova EA, Cinelli P. The role of the leukemia inhibitory factor (LIF)—pathway in derivation and maintenance of murine pluripotent stem cells. Genes. 2011;2:280–297. doi: 10.3390/genes2010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Zhao H, Yenari MA, Sapolsky RM, Steinberg GK. Catalase over-expression protects striatal neurons from transient focal cerebral ischemia. NeuroReport. 2004;15:413–416. doi: 10.1097/00001756-200403010-00006. [DOI] [PubMed] [Google Scholar]
- Guevara R, Santandreu FM, Valle A, Gianotti M, Oliver J, Roca P. Sex-dependent differences in aged rat brain mitochondrial function and oxidative stress. Free Radical Biology and Medicine. 2009;46:169–175. doi: 10.1016/j.freeradbiomed.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Ishibashi N, Prokopenko O, Weisbrot-Lefkowitz M, Reuhl KR, Mirochnitchenko O. Glutathione peroxidase inhibits cell death and glial activation following experimental stroke. Mol Brain Res. 2002;109:34–44. doi: 10.1016/s0169-328x(02)00459-x. [DOI] [PubMed] [Google Scholar]
- Khanna A, Kahle KT, Walcott BP, Gerzanich V, Simard JM. Disruption of Ion Homeostasis in the Neurogliovascular Unit Underlies the Pathogenesis of Ischemic Cerebral Edema. Translational Stroke Res. 2014;5:3–16. doi: 10.1007/s12975-013-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd PM. Glutathione: systemic protectant against oxidative and free radical damage. Altern Med Rev. 1997;2:155–176. [Google Scholar]
- Kim GW, Kondo T, Noshita N, Chan PH. Manganese Superoxide Dismutase Deficiency Exacerbates Cerebral Infarction After Focal Cerebral Ischemia/Reperfusion in Mice Implications for the Production and Role of Superoxide Radicals. Stroke. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Translational Stroke Res. 2013;4:390–401. doi: 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Laterza C, Merlini A, De Feo D, Ruffini F, Menon R, Onorati M, Fredrickx E, Muzio L, Lombardo A, Comi G, Quattrini A, Taveggia C, Farina C, Cattaneo E, Martino G. iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat Commun. 2013;4:2597. doi: 10.1038/ncomms3597. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PloS One. 2007;2:e156. doi: 10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Kim JH, Palmiter RD, Koh JY. Zinc released from metallothionein-iii may contribute to hippocampal CA1 and thalamic neuronal death following acute brain injury. Exp Neuro. 2003;184:337–347. doi: 10.1016/s0014-4886(03)00382-0. [DOI] [PubMed] [Google Scholar]
- Lee PC, Salyapongse AN, Bragdon GA, Shears LL, Watkins SC, Edington HDJ, Billiar TR. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physi-Heart Circ Physi. 1999;277:H1600–H1608. doi: 10.1152/ajpheart.1999.277.4.H1600. [DOI] [PubMed] [Google Scholar]
- Leonardo CC, Musso J, Das M, Rowe DD, Collier LA, Mohapatra S, Pennypacker KR. CCL20 is associated with neurodegeneration following experimental traumatic brain injury and promotes cellular toxicity in vitro. Translational Stroke Research. 2012;3:357–363. doi: 10.1007/s12975-012-0203-8. [DOI] [PubMed] [Google Scholar]
- Li P, Hu X, Gan Y, Gao Y, Liang W, Chen J. Mechanistic insight into DNA damage and repair in ischemic stroke: exploiting the base excision repair pathway as a model of neuroprotection. Antioxidants & Redox Signaling. 2011;14:1905–1918. doi: 10.1089/ars.2010.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TH, Beckman JS, Freeman BA, Hogan EL, Hsu CY. Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Phys Heart Circ Physiol. 1989;256:H589–H593. doi: 10.1152/ajpheart.1989.256.2.H589. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Ehmann WD, Butler SM, Markesbery WR. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology. 1995;45:1594–1601. doi: 10.1212/wnl.45.8.1594. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurology. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Lukic-Panin V, Deguchi K, Yamashita T, Shang J, Zhang X, Tian F, Liu N, Kawai H, Matsuura T, Abe K. Free radical scavenger edaravone administration protects against tissue plasminogen activator induced oxidative stress and blood brain barrier damage. Curr Neurovasc Res. 2010;7:319–329. doi: 10.2174/156720210793180747. [DOI] [PubMed] [Google Scholar]
- Madhavan L, Ourednik V, Ourednik J. Neural stem/progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. STEM CELLS. 2008;26:254–265. doi: 10.1634/stemcells.2007-0221. [DOI] [PubMed] [Google Scholar]
- Martínez-Serrano A, Björklund A. Protection of the neostriatum against excitotoxic damage by neurotrophin-producing, genetically modified neural stem cells. J Neurosci. 1996;16:4604–4616. doi: 10.1523/JNEUROSCI.16-15-04604.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Martinou I, Kato AC. Cholinergic differentiation factor (CDF/LIF) promotes survival of isolated rat embryonic motoneurons in vitro. Neuron. 1992;8:737–744. doi: 10.1016/0896-6273(92)90094-t. [DOI] [PubMed] [Google Scholar]
- Masters BA, Quaife CJ, Erickson JC, Kelly EJ, Froelick GJ, Zambrowicz BP, Brinster RL, Palmiter RD. Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J Neurosci. 1994;14:5844–5857. doi: 10.1523/JNEUROSCI.14-10-05844.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SL, Lin Y, Chen W, Yu F, Cao L, He Q, Chan PH, Li PA. Manganese superoxide dismutase deficiency exacerbates ischemic brain damage under hyperglycemic conditions by altering autophagy. Translational Stroke Res. 2011;2:42–50. doi: 10.1007/s12975-010-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni G, Sonois V, Delaine T, Guilloreau L, Gillet A, Teissié J, Faller P, Vašák M. Metal swap between Zn7-metallothionein-3 and amyloid-β–Cu protects against amyloid-β toxicity. Nature Chem Bio. 2008;4:366–372. doi: 10.1038/nchembio.89. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunology. 1993;151:2132–2141. [PubMed] [Google Scholar]
- Mikawa S, Li Y, Huang T, Carlson E, Chen S, Kondo T, Murakami K, Epstein C, Chan P. Cerebral infarction is exacerbated in mitochondrial manganese superoxide dismutase (Sod-2) knockout mutant mice after focal cerebral ischemia and reperfusion. Soc Neurosci Abstr. 1995;21:1268. [Google Scholar]
- Moncada S, Higgs A. The L-Arginine-Nitric Oxide Pathway. New England J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Yu J, Ng R, Johnson DA, Shen H, Honey CR, Johnson JA. Preferential expression of antioxidant response element mediated gene expression in astrocytes. J Neurochem. 2001;76:1670–1678. doi: 10.1046/j.1471-4159.2001.00157.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TLX, Kim CK, Cho JH, Lee KH, Ahn JY. Neuroprotection signaling pathway of nerve growth factor and brain-derived neurotrophic factor against staurosporine induced apoptosis in hippocampal H19-7 cells. Exp Mol Med. 2010;42:583–595. doi: 10.3858/emm.2010.42.8.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel CH, Ruedinger J, Misch F, Blume K, Maile S, Schulte J, Köhrle J, Hartmann O, Giersdorf S, Bingisser R. Copeptin and Peroxiredoxin-4 Independently Predict Mortality in Patients With Nonspecific Complaints Presenting to the Emergency Department. Academic Emergency Medicine. 2011;18:851–859. doi: 10.1111/j.1553-2712.2011.01126.x. [DOI] [PubMed] [Google Scholar]
- Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, Yamauchi-Takihara K. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem. 1998;273:9703–9710. doi: 10.1074/jbc.273.16.9703. [DOI] [PubMed] [Google Scholar]
- Okado-Matsumoto A, Matsumoto A, Fujii J, Taniguchi N. Peroxiredoxin IV is a secretable protein with heparin-binding properties under reduced conditions. J Biochem. 2000;127:493–501. doi: 10.1093/oxfordjournals.jbchem.a022632. [DOI] [PubMed] [Google Scholar]
- Özgönül M, Öge A, Sezer ED, Bayraktar F, Sözmen EY. The effects of estrogen and raloxifene treatment on antioxidant enzymes in brain and liver of ovarectomized female rats. Endocrine Res. 2003;29:183–189. doi: 10.1081/erc-120022299. [DOI] [PubMed] [Google Scholar]
- Pennypacker KR. Targeting the peripheral inflammatory response to stroke: role of the spleen. Translational Stroke Research. 2014;5:635. doi: 10.1007/s12975-014-0372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo G, Matera M, Casanova G, Ruggiero F, Paradies G. Mitochondrial dysfunction in rat brain with aging: involvement of complex I, reactive oxygen species and cardiolipin. Neurochemistry international. 2008;53:126–131. doi: 10.1016/j.neuint.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Pompella A, Visvikis A, Paolicchi A, de Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharm. 2003;66:1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- Raps SP, Lai JCK, Hertz L, Cooper AJL. Glutathione is present in high concentrations in cultured astrocytes but not in cultured neurons. Brain Res. 1989;493:398–401. doi: 10.1016/0006-8993(89)91178-5. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Woo HA, Kil IS, Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem. 2012;287:4403–4410. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario de la Torre M, Casado A, López-Fernández M, Carrascosa D, Casado M, Venarucci D, Venarucci V. Human aging brain disorders: Role of antioxidant enzymes. Neurochem Res. 1996;21:885–888. doi: 10.1007/BF02532336. [DOI] [PubMed] [Google Scholar]
- Rowe DD. Secreted Factors from Human Umbilical Cord Blood Cells Protect Oligodendrocytes from Ischemic Insult 2011 [Google Scholar]
- Rowe DD, Leonardo CC, Hall AA, Shahaduzzaman MD, Collier LA, Willing AE, Pennypacker KR. Cord blood administration induces oligodendrocyte survival through alterations in gene expression. Brain Res. 2010;1366:172–188. doi: 10.1016/j.brainres.2010.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DD, Leonardo CC, Recio JA, Collier LA, Willing AE, Pennypacker KR. Human umbilical cord blood cells protect oligodendrocytes from brain ischemia through Akt signal transduction. J Biol Chem. 2012;287:4177–4187. doi: 10.1074/jbc.M111.296434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DR, Collier LA, Seifert HA, Chapman CB, Leonardo CC, Willing AE, Pennypacker KR. Leukemia inhibitory factor promotes functional recovery and oligodendrocyte survival in rat models of focal ischemia. Eur J Neurosci. 2014;40:3111–3119. doi: 10.1111/ejn.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrave AM, Mager DE, Charman SA, Edwards GA, Porter CJH. Pharmacokinetics of Recombinant Human Leukemia Inhibitory Factor in Sheep. J Pharm Exp Therap. 2004;309:1085–1092. doi: 10.1124/jpet.103.063289. [DOI] [PubMed] [Google Scholar]
- Seifert HA, Pennypacker KR. Molecular and celluar immune responses to ischemic brain injury. Translantional Stroke Res. 2014;5:543–553. doi: 10.1007/s12975-014-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y, Yang SR, Jee MK, Joo EK, Roh KH, Seo MS, Han TH, Lee SY, Ryu PD, Jung JW. Human umbilical cord blood-derived mesenchymal stem cells protect against neuronal cell death and ameliorate motor deficits in Niemann Pick type C1 mice. Cell Transpl. 2011;20:1033–1047. doi: 10.3727/096368910X545086. [DOI] [PubMed] [Google Scholar]
- Shahaduzzaman M, Mehta V, Golden JE, Rowe DD, Green S, Tadinada R, Foran E, Sanberg PR, Pennypacker KR, Willing AE. Human umbilical cord blood cells induce neuroprotective change in gene expression profile in neurons afteriIschemia through activation of Akt pathway. Cell Transplant. 2015 doi: 10.3727/096368914X685311. (Epub ahead of press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Brady TC, Pearlstein RD, Crapo JD, Warner DS. Extracellular superoxide dismutase deficiency worsens outcome from focal cerebral ischemia in the mouse. Neurosci Lett. 1999;267:13–16. doi: 10.1016/s0304-3940(99)00316-x. [DOI] [PubMed] [Google Scholar]
- Shenoda B. The Role of Na+/Ca2+ Exchanger Subtypes in Neuronal Ischemic Injury. Translational Stroke Res. 2015;6:181–190. doi: 10.1007/s12975-015-0395-9. [DOI] [PubMed] [Google Scholar]
- Shi Y, Leak RK, Keep RF, Chen J. Translational Stroke Research on Blood-Brain Barrier Damage: Challenges, Perspectives, and Goals. Translational Stroke Res. 2016;7:89–92. doi: 10.1007/s12975-016-0447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H, Kitazono T, Yanagawa T. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nature Med. 2012;18:911–917. doi: 10.1038/nm.2749. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T, Wasiewski WW, Emeribe U. NXY-059 for the treatment of acute ischemic stroke. New England Journal of Medicine. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- Sobočanec S, Balog T, Šverko V, Marotti T. Sex-dependent Antioxidant Enzyme Activities and Lipid Peroxidation in Ageing Mouse Brain. Free Radical Res. 2003;37:743. doi: 10.1080/1071576031000102178. [DOI] [PubMed] [Google Scholar]
- Stahl N, Boulton TG, Farruggella T, Ip NY, Davis S, Witthuhn BA, Quelle FW, Silvennoinen O, Barbieri G, Pellegrini S, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Rotter S, Wassmann S, Adam O, Grohé C, Laufs K, Böhm M, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circulation research. 2003;93:170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- Strid S, Borga O, Edenius C, Jostell KG, Odergren T, Weil A. Pharmacokinetics in renally impaired subjects of NXY-059, a nitrone-based, free-radical trapping agent developed for the treatment of acute stroke. Eur J Clin Pharmacol. 2002;58:409–415. doi: 10.1007/s00228-002-0478-x. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Vašák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- Tomida M, Yamamoto-Yamaguchi Y, Hozumi M. Purification of a factor inducing differentiation of mouse myeloid leukemic M1 cells from conditioned medium of mouse fibroblast L929 cells. J Biol Chem. 1984;259:10978–10982. [PubMed] [Google Scholar]
- Vainshtein BK, Melik-Adamyan WR, Barynin VV, Vagin AA, Grebenko AI. Three-dimensional structure of the enzyme catalase. Nature. 1981;293:411–412. doi: 10.1038/293411a0. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Gemma C, De Mesquita D, Collier L, Bickford PC, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- Weisbrot-Lefkowitz M, Reuhl K, Perry B, Chan PH, Inouye M, Mirochnitchenko O. Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Mol Brain Res. 1998;53:333–338. doi: 10.1016/s0169-328x(97)00313-6. [DOI] [PubMed] [Google Scholar]
- Wen TC, Rogido MR, Moore JE, Genetta T, Peng H, Sola A. Cardiotrophin-1 protects cortical neuronal cells against free radical-induced injuries in vitro. Neurosci Letters. 2005;387:38–42. doi: 10.1016/j.neulet.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Wiener H, Perry R, Chen Z, Harrell L, Go R. A polymorphism in SOD2 is associated with development of Alzheimer’s disease. Genes, Brain and Behavior. 2007;6:770–776. doi: 10.1111/j.1601-183X.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Ebselen in Acute Ischemic Stroke: A Placebo-Controlled, Double-blind Clinical Trial. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- Yamamori T, Fukada K, Aebersold R, Korsching S, Fann MJ. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science. 1989;246:1412. doi: 10.1126/science.2512641. [DOI] [PubMed] [Google Scholar]
- You HJ, Oh D, Choi CY, Lee DG, Hahm KS, Moon AR, Jeong HG. Protective effect of metallothionein-III on DNA damage in response to reactive oxygen species. Biochimica et Biophysica Acta (BBA)-General Subjects. 2002;1573:33–38. doi: 10.1016/s0304-4165(02)00325-2. [DOI] [PubMed] [Google Scholar]
- Yu WH, Lukiw WJ, Bergeron C, Niznik HB, Fraser PE. Metallothionein III is reduced in Alzheimer’s disease. Brain Res. 2001;894:37–45. doi: 10.1016/s0006-8993(00)03196-6. [DOI] [PubMed] [Google Scholar]
- Zelko I, Folz R. Myeloid zinc finger (MZF)-like, Kruppel-like and Ets families of transcription factors determine the cell-specific expression of mouse extracellular superoxide dismutase. Biochem J. 2003;369:375–386. doi: 10.1042/BJ20021431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelko IN, Folz RJ. Sp1 and Sp3 transcription factors mediate trichostatin A-induced and basal expression of extracellular superoxide dismutase. Free Radical Biology and Medicine. 2004;37:1256–1271. doi: 10.1016/j.freeradbiomed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biology and Medicine. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Meta. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zivin JA. Acute stroke therapy with tissue plasminogen activator (tPA) since it was approved by the U.S. Food and Drug Administration (FDA) Annals of Neurology. 2009;66:6–10. doi: 10.1002/ana.21750. [DOI] [PubMed] [Google Scholar]