Figure 2. Identification of phosphorylation sites for the substrates of symbiotic receptor-like kinases.
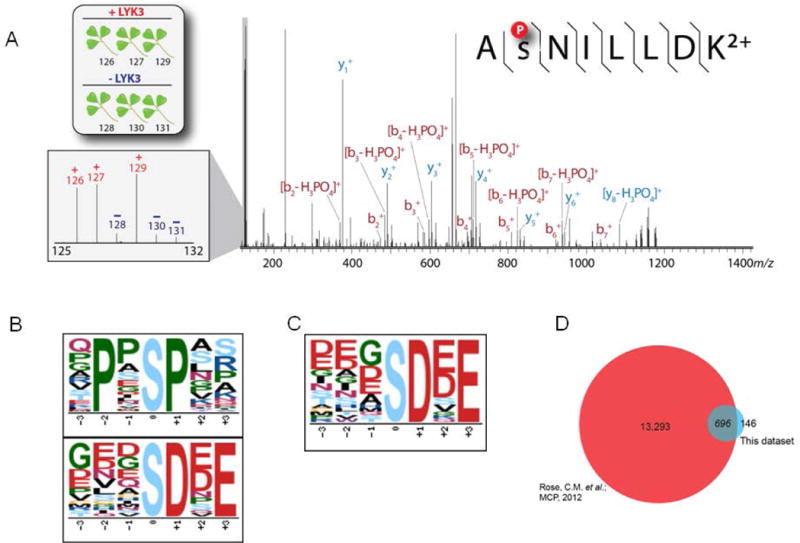
(A) Tandem mass spectrum of the phosphorylated peptide to AsNILLDK2+, a tryptic peptide of cysteine-rich receptor-kinase-like protein. Reporter tags are present in the low mass region (inset). The intensity of these reporter tags can be used to determine the amount of kinase-directed phosphorylation. Here, the phosphorylation intensity of the LYK3 treated channels (126, 127 and 129 m/z) show an increase in abundance compared to the control channels (128, 130, 131 m/z). Motif analysis of Medicago lysate treated with either (B) LYK3 or (C) NORK purified kinase. (D) Comparison of phosphopeptides from our previous work to the current work. Previously we identified 13293 phosphopeptides specific to symbiotic signaling in Medicago (Rose et al., 2012) and the current studies a total of 842 phosphopeptides were identified out of which 696 overlapped with our previous work and 146 were unique to this study.
