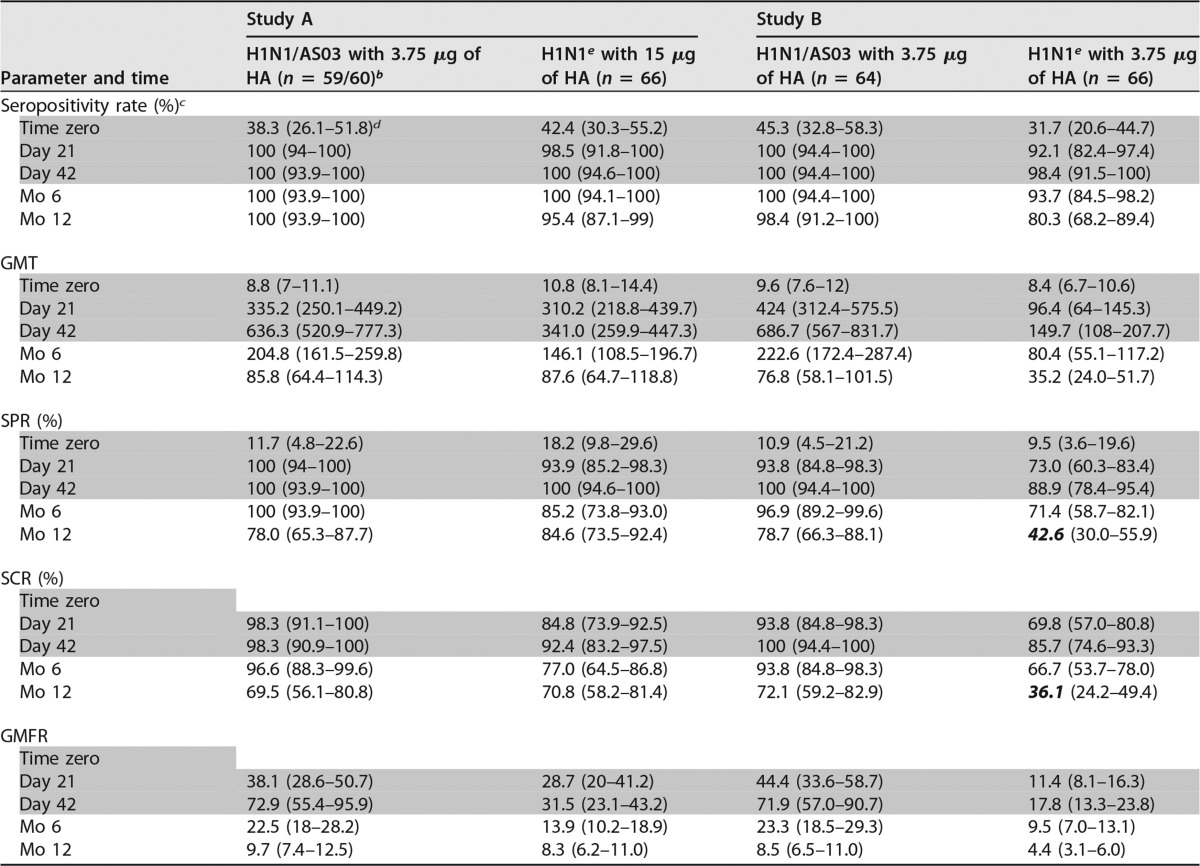
TABLE 2.
A(H1N1)pdm09-specific HI responses before and after vaccination up to month 12a
Gray-shaded data were reported previously (6) and are shown here for completeness. The data are from the per-protocol cohorts for immunogenicity at day 42, month 6, and month 12. Bold italic values are those that do not meet the European Medicines Agency CHMP criteria for HI antibody responses in adults 18 to 60 years old, i.e., an SPR of >70%, an SCR of >40%, and a GMFR of >2.5.
b n = 60 for time zero and day 21; n = 59 for all subsequent time points.
c The seropositivity rate is the percentage of subjects with HI titers within the specified range.
d Values in parentheses are 95% CIs.
e H1N1, A(H1N1)pdm09 vaccine.