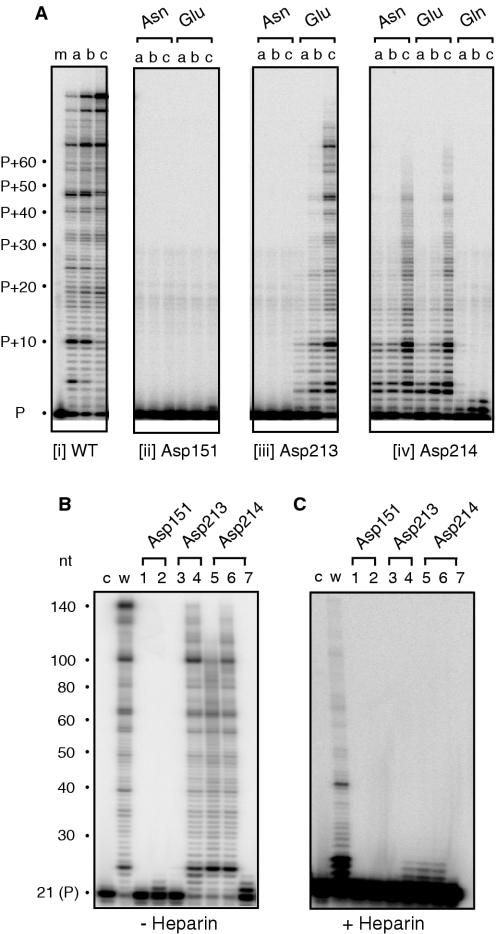
Figure 1.
RNA-dependent DNA polymerase activity of Ty3 DNA polymerase active site mutants. (A) An aliquot of 50 nM template/primer was incubated with 0.8 nM (lane a), 5 nM (lane b) and 35 nM Ty3 RT (lane c) for 5 min, respectively. The active site residues analyzed and nature of the mutation are indicated below and above each panel, respectively. P, unextended primer. (B) RNA-dependent DNA polymerase activity in the absence of competitor heparin. For this analysis, 50 nM template/primer was incubated with 200 nM RT for 5 min. Lane c, 32P-end-labeled primer; lane w, wild-type Ty3 RT; lane 1, Asp151→Asn; lane 2, Asp151→Glu; lane 3, Asp213→Asn; lane 4, Asp213→Glu; lane 5, Asp214→Asn; lane 6, Asp214→Glu; and lane 7, Asp214→Gln. (C) RNA-dependent DNA polymerase activity in the presence of competitor heparin. Lane notations are as in (B).