Abstract
Background/Aim: As an alternative material to the autogenous bone, duck-beak bone particle for bone substitute have been attracting great attention due to their biological properties. To deliver the most favorable outcome of medical treatment, it is essential to study the effect of various processing methods of the duck-beak bone. In this study, we compared the two deproteinizing agents for manufacturing duck-beak bone. Group 1 was treated by a conventional chemical agent (ethylenediamine) and Group 2 by hydrogen dioxide (H2O2). In vitro and in vivo experiments were conducted in parallel to compare the cytocompatibility and osteogenic capability between two processing methods. For in vitro tests, human adipose-derived mesenchymal stem cells (hAD-MSCs) were planted onto each sample and their attachment and growing were evaluated. For in vivo biocompatibility and osteogenic properties, the samples were applied on the critical-sized calvarial bone defect of rats. Group 2 showed significantly higher cell attachment but Group1 showed slightly higher cell proliferation. In in vivo tests, all groups have shown biocompatibility and increased level of osteogenic potential. However, Group 2 had significantly higher bone regeneration (p<0.05). This experiment confirmed that H2O2 can be an optimal processing method for duck-beak bone particle.
Keywords: Duck-beak, bone, osteogenic, potential, hydrogen dioxide
For a successful tissue-engineering strategy, it is required to select a suitable scaffold to maintain the space for tissue growth and regeneration. Powerful osteogenic potential of autogenous bone makes this graft be regarded as the “gold standard”; however, this graft has disadvantage in limited quantity and donor morbidity (1). It is, thus, required to find three-dimensional (3D), biodegradable and biocompatible scaffolds mimicking the bone tissue architecture for tissue engineering applications as an alternative (2,3). Although soft scaffolds (e.g., hydrogels or collagen sponge) have been predominantly used in the tissue engineering filed, these are limited by their poor mechanical properties. On the contrary, solid scaffolds (e.g., hydroxyapatite or tri-calcium phosphate) can provide improved support for tissue regeneration (4-6). The solid scaffolds initially support the formation of regenerative tissue into the defect area by preserving space (7). Finally, however, the scaffold is gradually replaced by regenerated tissue (8).
Livestock ducks are mainly used for food production with their heads usually discarded or used to produce natural organic fertilizer and animal feeds. The top part of the beak is called the upper mandible and the bottom part the lower mandible. The duck-beak is composed of keratin containing two major bones in each side of the lower mandible and upper mandible, with a small nose bone affixed to the skull. These wasted duck-beak bones have potentially valuable uses in biomaterial fields, especially in bone graft substitutes (9). Bone defects often occur as a result of trauma, bone tumors, resection and metabolic diseases. These defects are typically reconstructed using either a natural bone graft or an artificial synthetic bone graft. As an alternative material to the autogenous bone, duck-beak bone particles, which are natural hydroxyapatite particles for bone substitute, have been attracting great attention. Due to their biological properties, such as high porosity, duck-beak bone has high affinity to osteoblast and biocompatibility (9). To deliver the most favorable outcome of the medical treatment, it is essential to study the effect of various processing methods of the duck-beak bone.
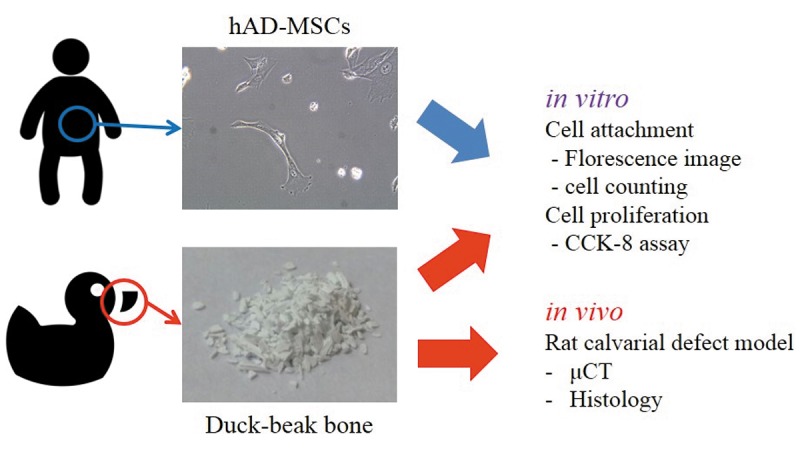
In this study, we compared samples by their osteogenic potential to verify which processing method makes the best bone capacity. The main comparative subject was deproteinizing chemical agents. Two kinds of chemical agents were used. One agent, ethylenediamine (EDA), is conventionally used in deproteinizing processes. The other is H2O2, which is a natural substance. In vitro and in vivo experiments were conducted in parallel to compare the cytocompatibility and osteogenic capability between the two processing methods (Figure 1).
Figure 1. The optical image of the duck-beak bone microparticles and microscopic image of the human adipose-derived mesenchymal stem cells of this study are shown. hAD-MSCs, human adipose-derived mesenchymal stem cells; μC, micro-computed tomography.
Materials and Methods
Duck-beak bone particle preparation. Duck-beak bones were surgically extracted and all soft tissues removed. The collected bone samples were boiled with distilled water for 8 hours and then deproteinized. Group 1 samples were deproteinized with EDA and Group 2 samples with H2O2. Subsequently, each group was sintered at high temperature by an electric furnace. Groups 1 and 2 were processed 20 hours at 400˚C. Through the processes above, organic matrix and disease-causing agents were all removed from the samples. All duck-beak bone pieces were subsequently milled into particles of 500-700 μm (10). There were two different groups of the samples divided by the processing that the duck-beak bone particle had been taken.
In vitro cell culture conditions. Human adipose-derived mesenchymal stem cells (hAD-MSCs) were incubated with the duck-beak bone particle on control media and osteogenic media (Figure 2). The in vitro experimental protocol was as follows. Twenty-five milligram-samples from each group were transferred into 24-well plates and disinfected by 70% ethyl alcohol for 30 minutes. After alcohol was removed, samples were rinsed by phosphate-buffered saline (PBS), three times for 5 minutes. Prepared samples were treated with two different types of media, control media (α-modified Eagle’s medium with 10% fetal bovine serum, supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin) and osteogenic media (control media with 0.1 mmol/l dexamethasone, 10 mmol/l β-glycerophosphate, 50 mmol/l ascorbate). The cells were incubated with samples at 37˚C in a humidified atmosphere with 5% CO2. The cells were loaded with each media at 1×104 cells/well rate. Media was changed every three days (2,11).
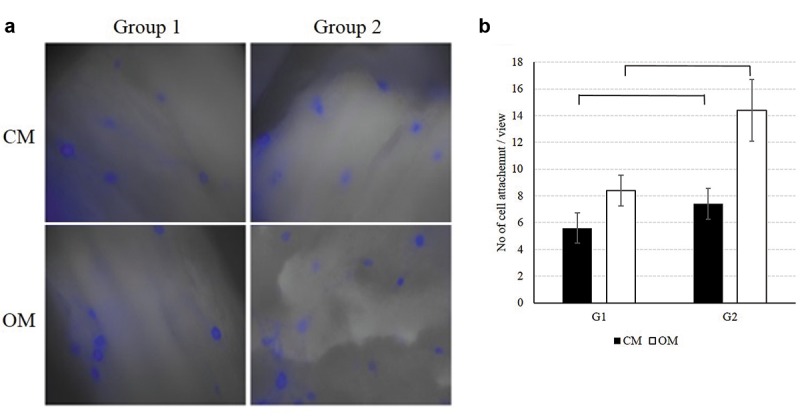
Figure 2. Cell attachment on the surface of duck-beak bone. Images of cell attachment were taken by a fluorescence microscope (a). Group 2 has a significantly higher number of attached cells compared with Group 1 (b) (×400). CM, Control media; OM, osteogenic media.
Cell attachment. The cells’ adhesive ability was compared by immunocytochemical staining. Fluorescence images of samples with control and osteogenic media were taken with a fluorescence microscope. Before samples were photographed, media was removed and remains were rinsed softly with PBS and fixed with 4% paraformaldehyde (PFA). After removing PFA, the samples were rinsed with PBS 2 times for 5 min. To increase cellular permeability, samples were processed with 1% Triton X-100 for 5 min. For blocking non-specific bonding, the samples were processed with 1% bovine serum albumin (BSA) for 30 min. Next, the samples were stained with Alexa Fluor 546-conjugated phalloidin (Invitrogen A22283; Invitrogen, Carlsbad, CA, USA) for 30 min. After rinsed with PBS twice, samples were processed with 0.1 μg/ml 4’,6’-diamidine-2’-phenylindole dihydrochloride (DAPI; Invitrogen P36935; Invitrogen) solution for 1 min (12). The results were photographed with a fluorescence microscope (IX71; Olympus, Tokyo, Japan) equipped with a digital camera.
Cell proliferation capacity. Cell proliferation capacity was tested at days 1, 3, 5 and 7 using the cell counting kit-8 (CCK-8; Dojindo, Molecular Technologies, Inc., Kumamoto, Japan). At each time point, cell culture media were removed from the samples and remains were rinsed softly with PBS twice. Two hundred μl of media and 20 μl of CCK-8 solution were added in each well and all together incubated for 2 h. After incubation, 100 μl of the eluted solution was transferred into 96-well plates and optical density (OD) was measured at 450 nm.
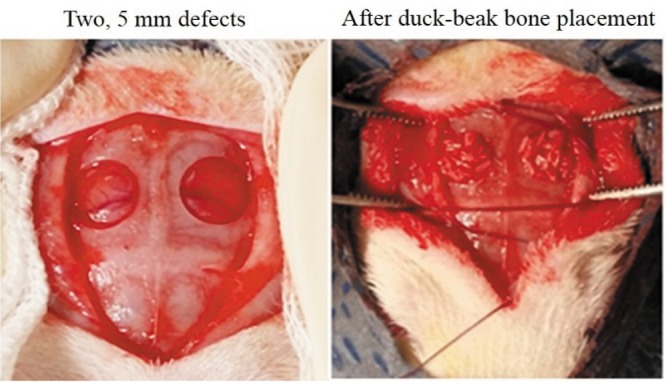
In vivo biocompatibility and osteogenic potential. In vivo experiments were conducted with 12 rats (n=6). Nine-week-old, male Sprague Dawley (Daehan Biolink Co., Ltd, Chungbuk, Korea) rats were used in this study. Animal experimentation was approved by the Institutional Animal Care and Use Committee of Chungbuk National University (no. 677-14-01). Groups’ 1 and 2 samples were sterilized before use. Both sides of calvarial bone of rats were holed 5 mm in size and then each sample was transplanted (1,13,14) (Figure 4). Twelve weeks after transplantation, calvarial bone tissue was collected and fixed with 10% neutral buffered formalin (NBF). The osteogenic potential was verified by micro-computed tomography (μCT) and histology.
Figure 4. In vivo critical-sized rat calvarial defect. After defect formation (Left), duck-beak bone particles are transplanted into the defect (Right).
Micro-computed tomographic analysis. The fixed specimens were imaged using an in vivo high resolution μCT system (Skyscan 1176; Skyscan, Aartselaar, Belgium). The samples were scanned at a pixel size of 12.56 μm, a filter of 1 mm aluminum, a rotation step of 0.5˚ and a rotation angle of 180˚. The X-ray tube voltage was 65 kV and the current 385 μA. Raw images were reconstructed by the NRecon Skyscan reconstruction software (Skyscan). Reconstructed images were used to analyze the percent of bone formation and to make two-dimensional (2D), as well as three-dimensional (3D) images.
Histological observation. After scanning μCT images, the tissues around the defect area were harvested and fixed in a 10% formalin solution for 24 hours at room temperature. Subsequently, the samples were dehydrated in an ascending series of alcohol rinses. Dehydrated samples were embedded in the acrylic resin Technovit 7200 VLC (Kulzer & Co GmbH, Friedrichsdorf, Germany) and serially cut into 20 μm-thick sections. The specimens were mounted on glass slides and stained with hematoxylin and eosin (H&E) stain, as well as Masson’s trichrome (MT) stain. Microscopic images were taken with a light-microscope.
Statistical analysis. The results are expressed as mean±standard deviation. For testing statistical significance between two groups, results were analyzed by one-way analysis of variance, followed by a Tukey’s test. Differences with p<0.05 were considered to be statistically significant.
Results
Cell attachment. After 3 weeks of incubation, the attached cells were stained and photographed with a fluorescence microscope. Well-attached hAD-MSCs were found in both control and osteogenic media at 400x (Figure 2A). The average value of the numbers of the attached cells were counted. In the control medium, Group 1 and Group 2 samples revealed 5.6±1.140 and 7.4±1.140 attached cells, respectively, whereas in the osteogenic medium, Group 1 and Group 2 samples gave 8.4±1.140 and 14.4±2.302 attached cells, respectively. Both in control and osteogenic media, Group 2 samples showed significantly higher cell attachment rate on the surface of particle compared with Group 1 (Figure 2B).
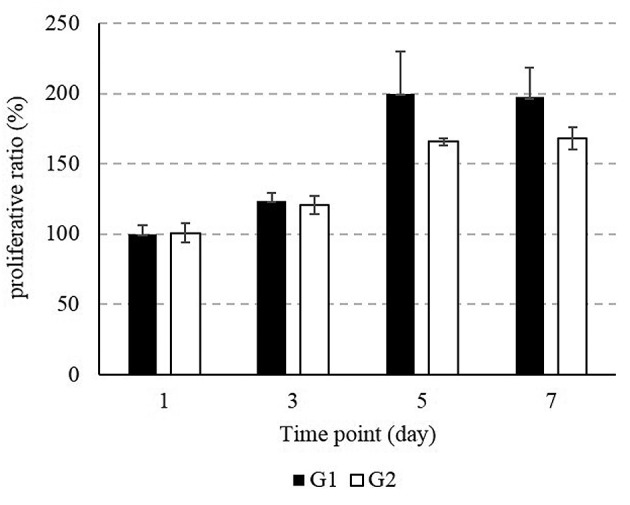
Cell proliferation capacity. Figure 3 shows the results of the CCK-8 assay. The cell proliferation capacity was slightly higher in Group 1, which was processed with EDA and heated at 400˚C for 20 h. The comparative growth rate of Group 1 samples on days 3, 5 and 7 of incubation was 123.8±5.4, 199.9±30.2 and 197±20.8, respectively, while the comparative growth rate of Group 2 samples at day 3, 5 and 7 of incubation was 120.5±6.4, 165.6±2.2 and 168.2±8.1, respectively. These results are shown in Figure 4 as a percent ratio. There was no significant difference on cell proliferation between the two groups.
Figure 3. Proliferative test on microparticles of both groups. The CCK- 8 assay confirms the cell proliferation capacity on days 1, 3, 5 and 7.
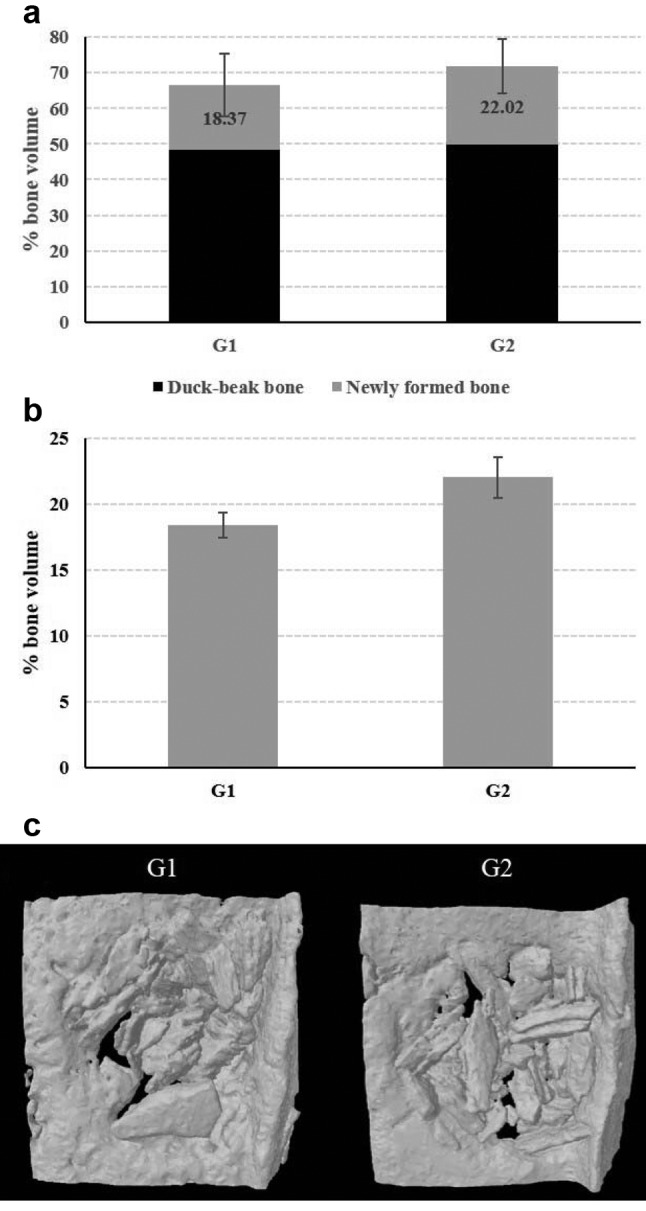
Micro-computed tomographic results of osteogenic potential. In vivo quantitative results of total new bone volume (including duck-beak bone and newly formed bone volume, Figure 5A), as well as newly formed bone, excluding duck-beak bone particle by thresholding (Figure 5B), were analyzed using μCT image analysis. The μCT-constructed images revealed new bone formation within the defect region of each experimental group. 3D images (Figure 5C) were made from reconstructed files. The total new bone volume in Group 1 and Group 2 was 66.97±8.77% and 72.30±7.61%, respectively; the effective percentage new bone volume of Group 1 and Group 2 was 18.37±0.95% and 22.02±1.52%, respectively. Total bone volume did not show any significant difference; however, after excluding the duck-beak bone particle from results, newly formed bone volume from Group 2 showed significantly higher osteogenic capacity compared with that of Group 1 in the μCT analysis.
Figure 5. In vivo critical-sized rat calvarial defect. After defect formation (Left), duck-beak bone particles are transplanted into the defect (Right).
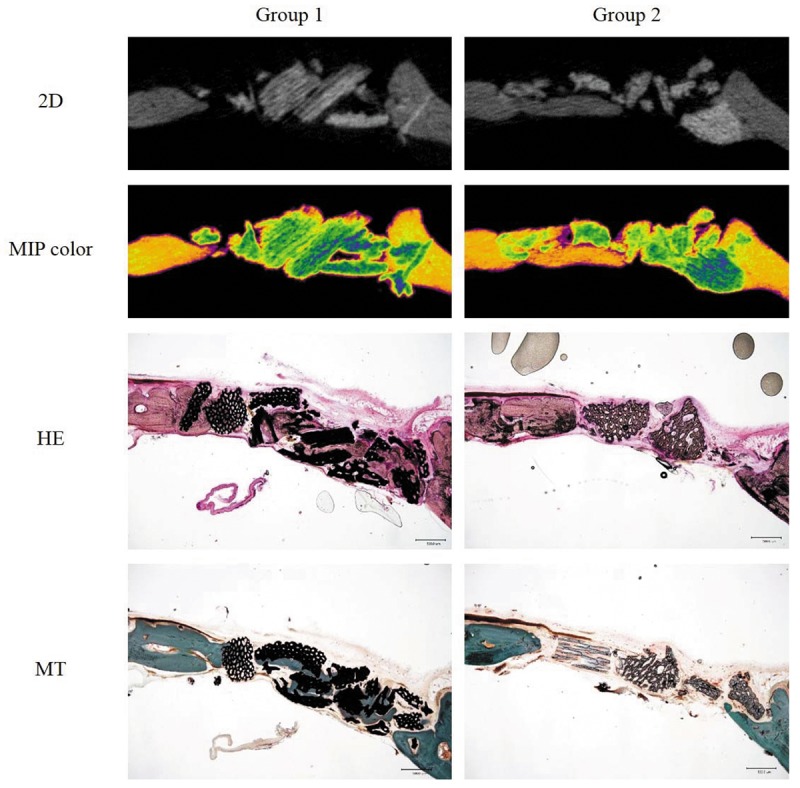
In vivo biocompatibility and histological findings. After H&E and MT staining, the microscopic images taken were compared with the cross sectional 2D and maximum intensity projection (MIP) μCT images (Figure 6). Bone formation was initiated from the periphery of host bone and newly formed bone was well integrated with the duck-beak bone particle in both Groups. Marked bone apposition was observed at the edge of the host bone to duck-beak bone particle interface. Importantly, in Groups 1 and 2, we could observe a number of osteoblasts and osteoclasts.
Figure 6. Cross sectional 2D micro-computed tomography (μ-CT) images of new bone formation at 12 weeks after duck-beak bone implantation in criticalsized calvarial bone defect in Sprague Dawley rats. Maximum intensity projection (MIP) images with color mapping help the visualization of new bone mineral density. Typical hematoxylin-eosin (H&E) and Masson’s trichrome (MT) stains of the non-decalcified sections are also presented (×40).
Discussion
In this study, in order to verify the optimum manufacture process of duck-beak bone particle to obtain the best osteogenic potential, we compared two groups with different types of deproteinizing agents. The agents were EDA, as conventional deproteinizing agent, and H2O2. To compare biocompatibility and osteogenic potential of two groups, in vitro cell attachment and proliferation capacity, as well as in vivo bone regeneration, were examined.
Use of scaffolds is an essential system to deliver stem cells to target areas and preserve space for the formation of newly growing tissue (7). For the present study, we used hAD-MSCs to verify cell attachment and proliferation capacity on the duck-beak bone particle, because AD-MSCs are well-established candidates for tissue engineering studies. AD-MSCs have several advantages over bone marrow-derived MSCs, such as large quantities of available stromal cells than bone marrow, easier and less invasive system for obtaining adipose tissue and similar characteristics to bone marrow-derived MSCs (11). Cell attachment was confirmed with direct visualization of stained hAD-MSCs, which grew on the duck-beak bone particle both in control and osteogenic media. Samples from both control and osteogenic media of Group 2 had significantly higher cell attachment rate. Both Group 1 and Group 2 exhibited a higher cell attachment rate on osteogenic media compared with control media. When human osteogenic precursors were cultured on a porous hydroxyapatite material, the cells exhibited an osteoblast-like morphology and a proliferation rate leading to confluent cultures (15).
For a successful bone tissue reconstruction on the critical bony defect, the use of 3D biodegradable and biocompatible synthetic and natural bone substitutes is an attractive alternative to autografts (1). These scaffolds should be suitable for maintaining the space for cell growth and differentiation (2). The proliferation, measured by the CCK-8 assay, showed that both groups exhibited an increase in hAD-MSCs’ numbers during the study periods. Especially, Group 1 showed slightly better cell growth than Group 2; however, the difference was not significant. Based on these promising findings, the in vivo osteogenic capacity of both scaffold systems was confirmed by conducting a bone regeneration study using the critical-sized rat calvarial defect model.
As an attractive alternative to the synthetic materials and allografts, xenografts have been shown having excellent osteoconductive properties and high biocompatibility (16). In an in vivo experiment, to confirm biocompatibility and osteogenic potential, transplanted duck-beak bone in critical-sized calvarial bone defect in Sprague Dawley rats was used. This animal model has been used to evaluate the biologic potential of various osteoinductive and/or osteoconductive graft materials to promote bone regeneration (17). Twelve weeks after transplantation, the defected region was scanned with μCT and newly formed bone volume was calculated. Both scaffolds showed significant new bone formation compared with empty control. However, Group 2 showed definitely outstanding osteogenic capacity. In histologic evaluation, the greatest amount of newly formed bone was present within the duck-beak bone microparticles. The results presented here also reveal that bone formation began from the periphery through the central part of the defect. Bio-Oss, a representative demineralized bone mineral (DBBM) from animal source, has proved its clinical availability as a xenograft for bone tissue engineering (18,19). These studies support our results, which showed that the duck-beak bone can induce bone cell ingrowth and osteogenic differentiation within the scaffold area.
This is the first comparative study on manufacturing duck-beak bone using two different deproteinizing agents to show that both EDA and H2O2 have potential for hard tissue regeneration. The analogous, or even superior, results of Group 2 suggest that the use of H2O2, as a deproteinizing agent, is valid and promising. However, other processing conditions, besides that of deproteinization, should additionally be considered to identify further potential advantages of using H2O2 as a deproteinizing agent.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A1A01060583) and the Next-Generation BioGreen 21 Program (PJ01135201), Rural Development Administration, Republic of Korea.
References
- 1.Khojasteh A, Eslaminejad MB, Nazarian H. Mesenchymal stem cells enhance bone regeneration in rat calvarial critical size defects more than platelete-rich plasma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:356–362. doi: 10.1016/j.tripleo.2007.10.017. discussion 363. [DOI] [PubMed] [Google Scholar]
- 2.Akita D, Morokuma M, Saito Y, Yamanaka K, Akiyama Y, Sato M, Mashimo T, Toriumi T, Arai Y, Kaneko T, Tsukimura N, Isokawa K, Ishigami T, Honda MJ. Periodontal tissue regeneration by transplantation of rat adipose-derived stromal cells in combination with PLGA-based solid scaffolds. Biomed Res (Tokyo, Japan) 2014;35:91–103. doi: 10.2220/biomedres.35.91. [DOI] [PubMed] [Google Scholar]
- 3.Shumilova AA, Myltygashev MP, Kirichenko AK, Nikolaeva ED, Volova TG, Shishatskaya EI. Porous 3D implants of degradable poly-3-hydroxybutyrate used to enhance regeneration of rat cranial defect. J Biomed Materials Res Part A. 2017;105:566–577. doi: 10.1002/jbm.a.35933. [DOI] [PubMed] [Google Scholar]
- 4.Nunez J, Sanz-Blasco S, Vignoletti F, Munoz F, Arzate H, Villalobos C, Nunez L, Caffesse RG, Sanz M. Periodontal regeneration following implantation of cementum and periodontal ligament-derived cells. J Periodontal Res. 2012;47:33–44. doi: 10.1111/j.1600-0765.2011.01402.x. [DOI] [PubMed] [Google Scholar]
- 5.Tobita M, Uysal AC, Ogawa R, Hyakusoku H, Mizuno H. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A. 2008;14:945–953. doi: 10.1089/ten.tea.2007.0048. [DOI] [PubMed] [Google Scholar]
- 6.Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, An Y, Zhang C, Wang S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem cells (Dayton, Ohio) 2010;28:1829–1838. doi: 10.1002/stem.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang HL, Greenwell H, Fiorellini J, Giannobile W, Offenbacher S, Salkin L, Townsend C, Sheridan P, Genco RJ. Research, Science and Therapy Committee (2005). Periodontal regeneration. J Periodontol. 2005;76:1601–1622. doi: 10.1902/jop.2005.76.9.1601. [DOI] [PubMed] [Google Scholar]
- 8.Rungsiyanont S, Dhanesuan N, Swasdison S, Kasugai S. Evaluation of biomimetic scaffold of gelatin-hydroxyapatite crosslink as a novel scaffold for tissue engineering: Biocompatibility evaluation with human PDL fibroblasts, human mesenchymal stromal cells, and primary bone cells. J Biomat Appl. 2012;27:47–54. doi: 10.1177/0885328210391920. [DOI] [PubMed] [Google Scholar]
- 9.Choi SH, Son JS, Kang SS, Lee CH. Production method for biomedical and industrial material using ceramic derived from birds’ beaks. US Patent App. 2012;14/360:169–169. [Google Scholar]
- 10.Son JS, Jang SH, Kwon TY, Kim KH, Kang SS, Choi SH. Preliminary evaluation of bone graft substitute produced by bone of duck beak. Materials Lett. 2014;121:181–184. [Google Scholar]
- 11.Akita D, Kano K, Saito-Tamura Y, Mashimo T, Sato-Shionome M, Tsurumachi N, Yamanaka K, Kaneko T, Toriumi T, Arai Y, Tsukimura N, Matsumoto T, Ishigami T, Isokawa K, Honda M. Use of rat mature adipocyte-derived dedifferentiated fat cells as a cell source for periodontal tissue regeneration. Front Physiol. 2016;7:50. doi: 10.3389/fphys.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Gaalen SM, Kruyt MC, Geuze RE, de Bruijn JD, Alblas J, Dhert WJ. Use of fluorochrome labels in in vivo bone tissue engineering research. Tissue Eng Part B Rev. 2010;16:209–217. doi: 10.1089/ten.TEB.2009.0503. [DOI] [PubMed] [Google Scholar]
- 13.Yoon E, Dhar S, Chun DE, Gharibjanian NA, Evans GR. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;133:619–627. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- 14.Slotte C, Lundgren D. Augmentation of calvarial tissue using non-permeable silicone domes and bovine bone mineral. An experimental study in the rat. Clin Oral Implants Res. 1999;10:468–476. doi: 10.1034/j.1600-0501.1999.100605.x. [DOI] [PubMed] [Google Scholar]
- 15.Faucheux C, Bareille R, Rouais F, Amédée J, Liebendörfer A, Dard M. Biocompatibility testing of a bovine hydroxyapatite ceramic material with the use of osteo-progenitor cells isolated from human bone marrow. J Mater Sci-Mater M. 1994;5:635–639. [Google Scholar]
- 16.Jensen SS, Broggini N, Weibrich G, Hjorting-Hansen E, Schenk R, Buser D. Bone regeneration in standardized bone defects with autografts or bone substitutes in combination with platelet concentrate: A histologic and histomorphometric study in the mandibles of minipigs. Int J Oral Maxillofac Implants. 2005;20:703–712. [PubMed] [Google Scholar]
- 17.Dahlin C, Alberius P, Linde A. Osteopromotion for cranioplasty. An experimental study in rats using a membrane technique. J Neurosurg. 1991;74:487–491. doi: 10.3171/jns.1991.74.3.0487. [DOI] [PubMed] [Google Scholar]
- 18.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa T, Ohgushi H, Okumura M, Tamai S, Dohi Y, Moriyama T. Biochemical and histological sequences of membranous ossification in ectopic site. Calcif Tissue Int. 1992;50:184–188. doi: 10.1007/BF00298798. [DOI] [PubMed] [Google Scholar]


