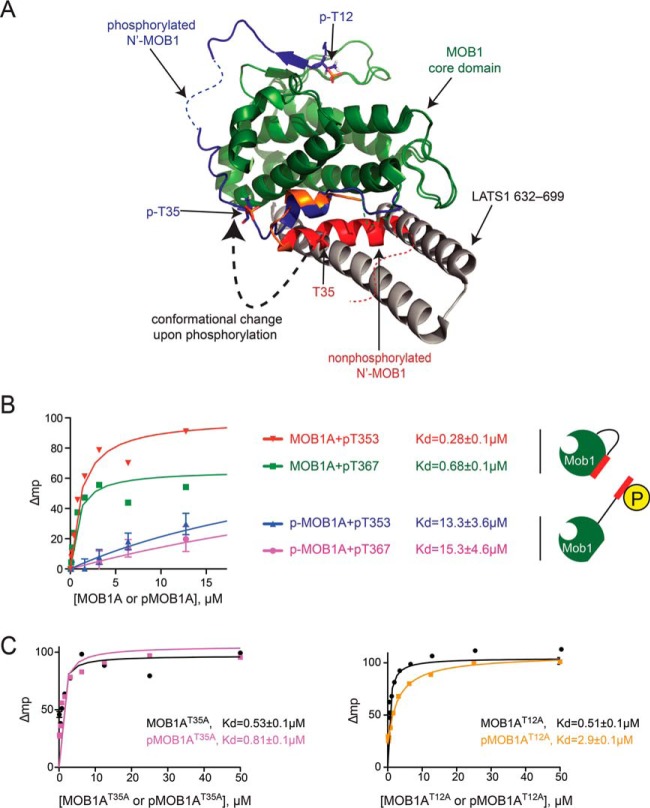
Fig. 3.
Phosphorylation on T12 and T35 induces switch-like conformational changes in MOB1A. A, Superimposition of full-length nonphosphorylated human MOB1A onto the human pMOB1B10–212 - LATS1602–704 complex (PDB 5BRK) (28). Colors for nonphosphorylated MOB1A are as in Fig. 2A. The N-terminal extension of pMOB1B10–212 is shown in blue; LATS1 in gray. B, Phosphorylation of MOB1A reduces its binding affinity for MST1 phosphopeptides. Fluorescence polarization analysis of MOB1A and phospho-MOB1A binding to MST1 pT353 and pT367 peptides. Data is plotted as the mean ± S.E. (n = 3). Note that the measurements for the unphosphorylated MOB1 protein are from (29). C, Phosphorylation of T12 and T35 sites in MOB1A collaborate to reduce binding affinity for MST1 phosphopeptides. Fluorescence polarization analysis of the binding of MOB1A mutants to MST1 phosphopeptides. Data is plotted as the mean ± S.E. (n = 3).