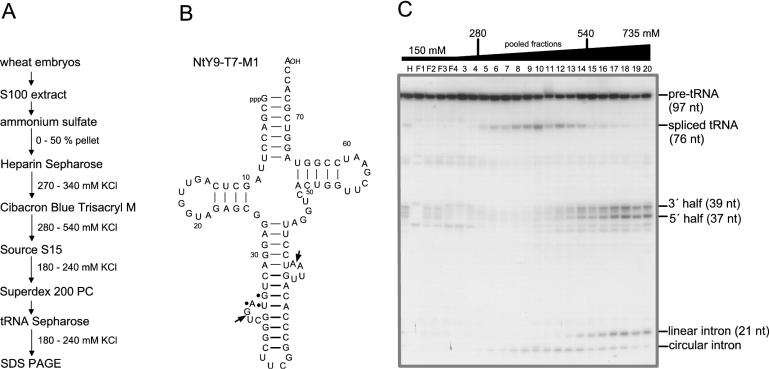
Figure 2.
Isolation of wheat germ tRNA ligase. (A) Purification scheme. RNA ligase was purified from the soluble protein fraction of wheat embryos (S100 extract) by six consecutive steps. (B) As substrate for assaying tRNA ligase activity, we have used a natural modified Nicotiana pre-tRNATyr (NtY9-T7-M1). The arrows in the two 4 nt bulge loops indicate the 3′ and 5′ splice sites and dots identify the anticodon. (C) Fractionation of wheat germ tRNA ligase by Cibacron Blue Trisacryl M chromatography and ligation activity assay. Partially purified tRNA ligase from the Heparin Sepharose column was applied onto a Blue-Trisacryl M column. Elution of tRNA ligase was performed with a gradient of 150–800 mM KCl. Fractions of 5 ml were collected. Splicing endonuclease co-eluted with tRNA ligase at this purification step, generating 3′ and 5′ tRNA halves. Reaction mixtures (20 μl) contained 20 mM Tris–HCl, pH 7.5, 6 mM Mg(OAc)2, 80 μM spermine, 1 mM ATP, 0.5 mM GTP, 0.1 mM DTT, 0.5% Triton X-100, 40 fmol (4 × 104 c.p.m.) of T7-transcript (NtY9-T7-M1) and 2 μl from eluted fractions. Incubation was for 30 min at 37°C. Products were analysed on a 12.5% polyacrylamide/8 M urea gel. tRNA ligase activity elutes between 280 and 540 mM KCl as revealed by the detection of mature, spliced tRNA.