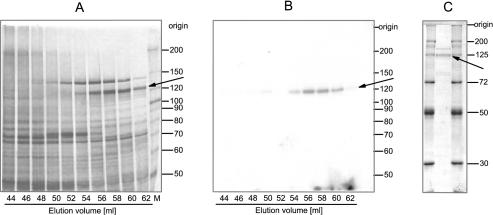
Figure 3.
Gel filtration on Superdex™ 200 and adenylyltransferase activity of wheat germ tRNA ligase. (A) Partially purified tRNA ligase from the Source S15 column was subjected to gel filtration on Superdex™ 200. The column (HiLoad™ 16/60) was run with a flow rate of 1 ml/min. Fractions of 2 ml were collected. Aliquots of the elution fraction were analysed on a 7.5% polyacrylamide/0.1% SDS gel. The proteins were visualized by silver staining. (B) Appropriate aliquots of the indicated fractions were incubated in the presence of [α-32P]ATP for 15 min at 37°C. The ligase–[32P]AMP adduct was detected by autoradiography of the dried gel. (C) The peak fractions from the tRNA Sepharose column were concentrated by ultrafiltration and 1/20 of this material was applied onto a 10% polyacrylamide/0.1% SDS gel and stained with Coomassie blue for analytical valuation. The arrows point to the position of the RNA ligase protein with an approximate molecular weight of 125 kDa. Protein size standards in kDa are indicated on the right.