Abstract
OBJECTIVE:
The aim of this study was to evaluate the effectiveness and safety of stem cell transplantation for spinal cord injury (SCI).
DATA SOURCES:
PubMed, EMBASE, Cochrane, China National Knowledge Infrastructure, China Science and Technology Journal, Wanfang, and SinoMed databases were systematically searched by computer to select clinical randomized controlled trials using stem cell transplantation to treat SCI, published between each database initiation and July 2016.
DATA SELECTION:
Randomized controlled trials comparing stem cell transplantation with rehabilitation treatment for patients with SCI. Inclusion criteria: (1) Patients with SCI diagnosed according to the American Spinal Injury Association (ASIA) International standards for neurological classification of SCI; (2) patients with SCI who received only stem cell transplantation therapy or stem cell transplantation combined with rehabilitation therapy; (3) one or more of the following outcomes reported: outcomes concerning neurological function including sensory function and locomotor function, activities of daily living, urination functions, and severity of SCI or adverse effects. Studies comprising patients with complications, without full-text, and preclinical animal models were excluded. Quality of the included studies was evaluated using the Cochrane risk of bias assessment tool and RevMan V5.3 software, provided by the Cochrane Collaboration, was used to perform statistical analysis.
OUTCOME MEASURES:
ASIA motor score, ASIA light touch score, ASIA pinprick score, ASIA impairment scale grading improvement rate, activities of daily living score, residual urine volume, and adverse events.
RESULTS:
Ten studies comprising 377 patients were included in the analysis and the overall risk of bias was relatively low level. Four studies did not detail how random sequences were generated, two studies did not clearly state the blinding outcome assessment, two studies lacked blinding outcome assessment, one study lacked follow-up information, and four studies carried out selective reporting. Compared with rehabilitation therapy, stem cell transplantation significantly increased the lower limb light touch score (odds ratio (OR) = 3.43, 95% confidence interval (CI): 0.01 – 6.86, P = 0.05), lower limb pinprick score (OR = 3.93, 95%CI: 0.74 – 7.12, P = 0.02), ASI grading rate (relative risk (RR) = 2.95, 95%CI: 1.64 – 5.29, P = 0.0003), and notably reduced residual urine volume (OR = –8.10, 95%CI: –15.09 to –1.10, P = 0.02). However, stem cell transplantation did not significantly improve motor score (OR = 1.89, 95%CI: –0.25 to 4.03, P = 0.08) or activities of daily living score (OR = 1.12, 95%CI: –1.17 to 4.04, P = 0.45). Furthermore, stem cell transplantation caused a high rate of mild adverse effects (RR = 14.49, 95%CI: 5.34 – 34.08, P < 0.00001); however, these were alleviated in a short time.
CONCLUSION:
Stem cell transplantation was determined to be an efficient and safe treatment for SCI and simultaneously improved sensory and bladder functions. Although associated minor and temporary adverse effects were observed with transplanted stem cells, spinal cord repair and axon remyelination were apparent. More randomized controlled trials with larger sample sizes and longer follow-up times are needed to further validate the effectiveness of stem cell transplantation in the treatment of SCI.
Keywords: nerve regeneration, spinal cord injury, stem cells, cell transplantation, bone marrow mesenchymal stem cells, umbilical cord blood stem cells, neural stem cells, human embryonic stem cells, paraplegia, metaanalysis, neural regeneration
Introduction
Spinal cord injury (SCI) is a highly debilitating disease caused by trauma, resulting in dyskinesia, sensory disturbance, dysreflexia, and sphincter dysfunction. Presently, the incidence of SCI is approximately 27–83 per million in the US and 10–30 per million in Europe (Wyndaele and Wyndaele, 2006; Hyun and Kim, 2010). Worldwide, there are more than 200 million people living with sequelae after SCI, including paralysis, locomotor and sensory dysfunction, urinary incontinence, and gastrointestinal dysfunction, which seriously impact patient quality of life and pose a substantial burden on the patient's family, society, and the healthcare system (Baaj et al., 2010; Cao et al., 2011; Post and van Leeuwen, 2012; Nowrouzi et al., 2016). Thus, development of a therapy to cure SCI is urgent.
The pathological reactions of SCI are complicated and comprise two phases: the primary injury phase and secondary injury phase (Kramer et al., 2013; Pannek et al., 2016). Primary injury is caused by mechanical compression and contusion from the fractured and dislocated bone fragments and discs around the spinal cord (Rowland et al., 2008), resulting in ischemia, spinal cord swelling, neuronal damage, axon disruption, and membrane rupture (McDonald and Sadowsky, 2002; Becker and McDonald, 2012). The secondary injury phase is a series of cascade reactions containing ischemia, electrolyte imbalance, inflammation, excitotoxicity, oxidative stress, necrosis, and apoptosis (Beattie et al., 2002; Donnelly and Popovich, 2008; Genovese and Cuzzocrea, 2008; Rowland et al., 2008). Both primary and secondary injuries lead to extensive tissue destruction, axonotmesis, demyelination, Wallerian degeneration, syringomyelia, and glial scar formation, which are difficult to cure and achieve a good rehabilitation outcome (Ung et al., 2010; Min et al., 2011). Many therapies are applied in the clinical treatment of SCI, including surgery, drugs, and functional training; however none of these are optimal. Stem cell transplantation aims to bridge cysts and cavities, replace dead cells, and to create a favorable environment for axon regeneration and remyelination, and has become a promising treatment for SCI (Thuret et al., 2006).
Stem cell transplantation has been proven to effectively restore the spinal cord by regenerating and remyelinating damaged axons and improving locomotion and sensation in many preclinical SCI rat studies (Cao et al., 2010; Erceg et al., 2010; Volarevic et al., 2013; Wu et al., 2015; Ide and Kanekiyo, 2016). The transplanted stem cells are able to survive, differentiate, integrate, and restore damaged tissues, leading to prominent improvement of locomotor function of SCI rats (Mothe and Tator, 2008; Parr et al., 2008; Moreno-Manzano et al., 2009; Erceg et al., 2010; Ruff et al., 2012; Zhang et al., 2016). Furthermore, transplanted stem cells are regarded as neuroprotective as they secrete various neurotrophic factors and inhibit apoptosis to provide support to damaged neurons and axons, eventually improving sensory function and locomotor function (Sasaki et al., 2009; Kim et al., 2010). Transplanted stem cells are able to self-renew and proliferate within the host and migrate along the host's central nervous system to the lesion site. Here, they differentiate into oligodendrocytes, astrocytes, and neurons to secrete many neurotrophic factors that support axonal regeneration and cell survival and restore syringomyelia caused by necrosis and apoptosis of neurons (Sasaki et al., 2009; Kim et al., 2010; Sandner et al., 2012). Some techniques are commonly used to perform transplantation, such as localized-lesion transplantation, intravenous injection, and subarachnoid injection. Localized-lesion transplantation is the first approach to transplant cells into animals with SCI and has a higher transplantation efficiency and enhanced rehabilitation compared with intravenous injection (Vaquero et al., 2006). However, this approach is not suitable for human SCI patients as it may result in secondary injury, spinal cord tract damage, and infection. Intravenous injection is a more convenient way to transplant cells, however the transplanted cells cannot traverse the blood-spinal cord barrier to reach the lesion and repair the injured spinal cord. Although the blood-spinal cord barrier is destroyed temporarily after SCI, providing a time window of about 7 days for transplantation (Maikos and Shreiber, 2007), most SCI patients receiving a stem cell transplantation are affected by chronic SCI (Keirstead et al., 2005, Takeuchi et al., 2007). With a higher transplantation efficiency, subarachnoid injection enables transplanted cells to migrate to the lesion site more easily to promote functional recovery in the SCI rats (Ohta et al., 2004, Bakshi et al., 2006); although this approach may lead to cerebrospinal fluid leakage and cause headache. To date however, it remains unknown whether stem cells transplanted into humans with SCI have the same effect on regeneration and functional recovery as in rats, and whether they result in any adverse effects in the host, such as tumors, infection, immune reaction, or even death.
To evaluate the effects and safety of stem cell transplantation on SCI patients, the present study reviewed randomized controlled trials on stem cell transplantation in the treatment of SCI by evaluating outcomes including the American Spinal Injury Association (ASIA) motor score, ASIA light touch score, ASIA pinprick score, ASIA impairment scale (AIS) grading improvement rate, activities of daily living (ADL) score, residual urine volume, and adverse events.
Data and Methods
Protocol and registration
This meta-analysis was reported in accordance with the Preferred Reporting Items for Systematic Review and Meta Analysis (PRISMA) (Liberati et al., 2009) and was registered in the International Prospective Register of Systematic Reviews (No. CRD42016043140).
Search strategy
PubMed, EMBASE, Cochrane, China National Knowledge Infrastructure, China Science and Technology Journal, Wanfang, and SinoMed databases were systematically searched to select relevant studies published between database initiations and July 2016, without language restrictions. Medical subject headings, such as stem cell transplantation, cell transplantation, mesenchymal stromal cells, neural stem cells, human embryonic stem cells, cord blood stem cell transplantation, induced pluripotent stem cells, paraplegia, spinal cord injuries, and free words were applied to search related references with the restriction of human randomized controlled trials. All potentially eligible studies were reviewed and a manual search was performed using reference lists of critical references. The retrieval details from PubMed, EMBASE, and Cochrane are presented in Table 1.
Table 1.
Detailed trial search strategy in PubMed

Inclusion and exclusion criteria
Inclusion criteria
All studies comprised: (1) randomized controlled trials of patients with SCI; (2) patients with SCI diagnosed according to ASIA International standards for neurological classification of SCI (Kirshblum et al., 2011), including those that had undergone operations due to different disease conditions before stem cell transplantation; (3) patients with SCI that received only stem cell transplantation or stem cell transplantation combined with rehabilitation; (4) one or more of the following reported outcomes: outcomes concerning neurological function, including sensory function and locomotor function, ADL, urination function, and severity of SCI or adverse effects.
Exclusion criteria
Studies meeting any of the following criteria were excluded: (1) SCI patients with complications such as anemia, diabetes, or pneumonia; (2) full-text unavailable; (3) non-human studies.
Study selection, data extraction and extracted data
Study titles and abstracts were reviewed by two independent investigators (XF and JZW) to decide if they satisfied the inclusion criteria and the full-text of the included studies was searched for further analysis.
Data were extracted by the same two independent investigators (XF and JZW) and disagreements were resolved by a third investigator (LZ).
Researchers from the present study extracted the following data from each selected study: authors; study groups; participant age; gender ratio; cell type; treatment strategy; transplantation method; and relative outcome measurements, including ASIA motor score, ASIA light score, and ASIA pinprick score; residual urine volume; ADL score; AIS grading improvement rate; and incidence of adverse effects.
Literature quality evaluation
Quality of the included studies was evaluated by two independent investigators (XF and JZW) according to the Cochrane risk of bias assessment tool (Armijo-Olivo et al., 2012) concerning: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Any disagreement regarding data during extraction and analysis was discussed and resolved by the third investigator (LZ).
Outcome indicators
In the present meta-analysis, outcome indicators included ASIA motor score, light touch score, pinprick score, AIS grading improvement rate, ADL score, residual urine volume, and adverse events.
Statistical analysis
Using the RevMan V5.3 software (Cochrane Collaboration, Copenhagen, Denmark) provided by the Cochrane Collaboration, the present study analyzed the ASIA motor score, light touch score, pinprick score, ADL score, and residual urine volume as continuous variables with mean difference and odds ratio (OR) corresponding to 95% confidence interval (CI). For analysis of the AIS grading rate and adverse effects rate, an overall relative risk (RR) with corresponding 95%CI was calculated. The I2 index and Cochrane Q test were employed to evaluate statistical heterogeneity in these estimates. With values of I2 > 75% (Higgins et al., 2003) and P < 0.1, there was statistical heterogeneity between different studies and the random-effects model was used, otherwise the fixed-effects model was employed.
Results
Study selection
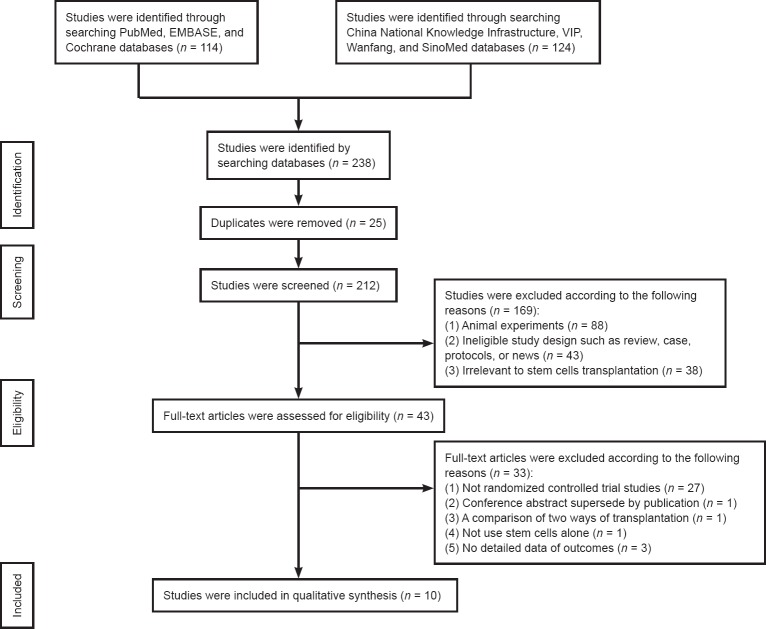
A total of 238 studies were initially identified following database searching. Among them, 10 studies (Xie et al., 2007; Cui et al., 2009; Fang et al., 2011; Guo et al., 2012; Li, 2012; Dai et al., 2013; Cheng et al., 2014; Guo, 2014; Xiao, 2014; Zhang et al., 2015) published between 2007 and 2015 comprising 377 patients were included in this final meta-analysis (Figure 1).
Figure 1.
Study selection process.
Study characteristics
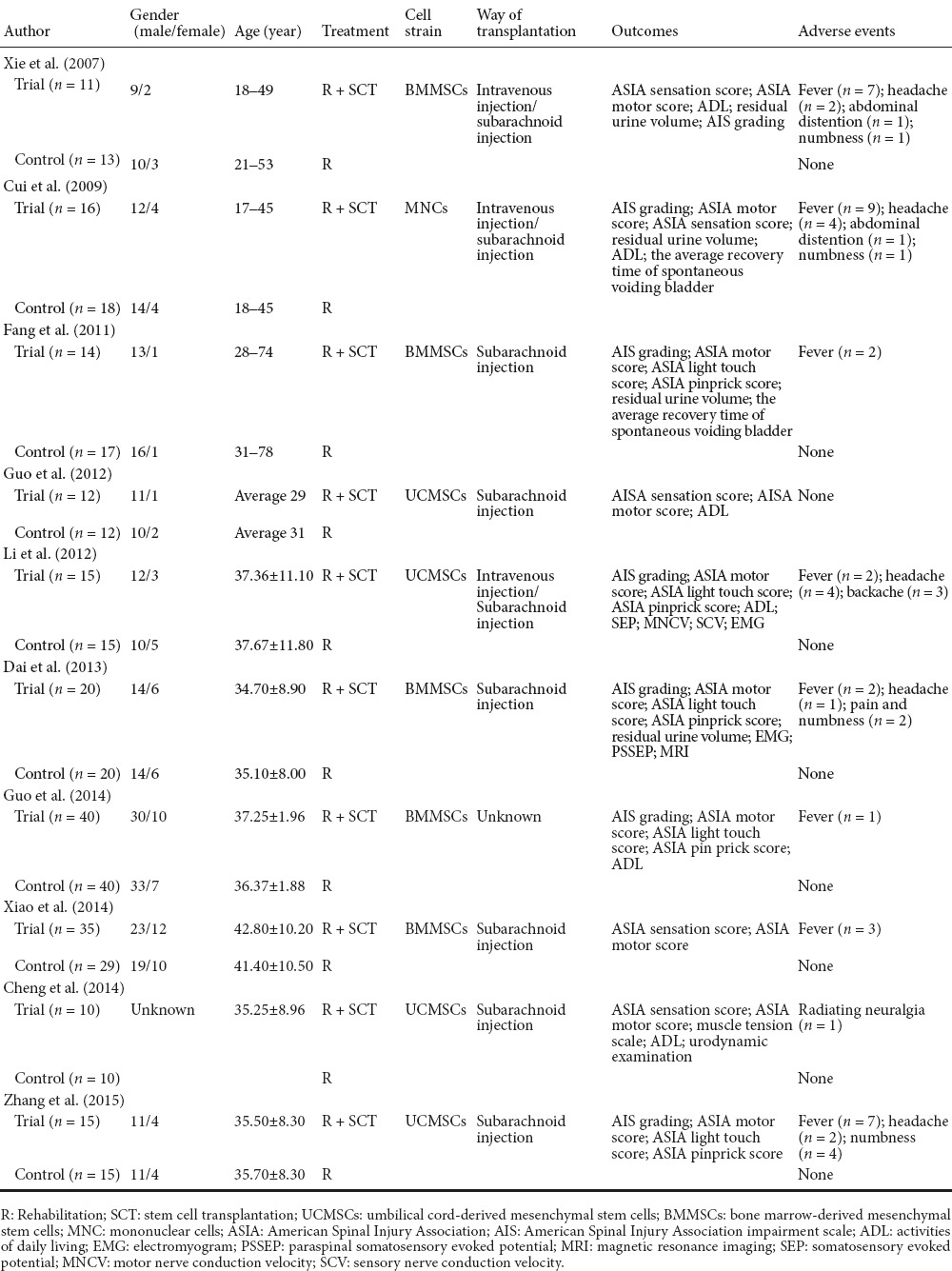
All 10 selected studies were performed in China and had a sample sizes ranging from 24 to 80 participants, with 3 studies published in English (Xie et al., 2007; Dai et al., 2013; Cheng et al., 2014). In most studies, patients suffered from chronic SCI, except in one study (Xiao, 2014) in which stem cells were transplanted into patients with acute SCI. All studies regarded rehabilitation therapy as the control group and the number of studies using umbilical cord-derived mesenchymal stem cells (Guo et al., 2012; Li, 2012; Cheng et al., 2014; Zhang et al., 2015) and bone marrow-derived mesenchymal stem cells for transplantation was five (Xie et al., 2007; Fang et al., 2011; Dai et al., 2013; Guo, 2014; Xiao, 2014). Only one study (Cui et al., 2009) transplanted mononuclear cells into the SCI patients. Six studies (Fang et al., 2011; Guo et al., 2012; Dai et al., 2013; Cheng et al., 2014; Xiao, 2014; Zhang et al., 2015) employed subarachnoid injection to transplant the stem cells, three studies (Xie et al., 2007; Cui et al., 2009; Li, 2012) employed both intravenous injection and subarachnoid injection, and one study (Guo, 2014) did not describe the transplantation method clearly. Trial follow-up periods ranged from 120 days to 12 months. In terms of outcome measurements, all 10 studies reported ASIA motor score (Xie et al., 2007; Cui et al., 2009; Fang et al., 2011; Guo et al., 2012; Li, 2012; Dai et al., 2013; Cheng et al., 2014; Guo, 2014; Xiao, 2014; Zhang et al., 2015); five studies reported ASIA light touch and pinprick score (Fang et al., 2011; Li, 2012; Dai et al., 2013; Guo, 2014; Zhang et al., 2015); six studies reported ADL score (Xie et al., 2007; Cui et al., 2009; Guo et al., 2012; Li, 2012; Cheng et al., 2014; Guo, 2014); five studies reported residual urine volume (Xie et al., 2007; Cui et al., 2009; Fang et al., 2011; Dai et al., 2013; Cheng et al., 2014); six studies evaluated AIS grading before and after intervention (Xie et al., 2007; Cui et al., 2009; Fang et al., 2011; Li, 2012; Dai et al., 2013; Zhang et al., 2015); and nine studies reported some mild adverse effects, such as fever, headache, pain, numbness, and abdominal distension; that were alleviated spontaneously or by treatments after intervention (Xie et al., 2007; Cui et al., 2009; Fang et al., 2011; Li, 2012; Dai et al., 2013; Cheng et al., 2014; Guo, 2014; Xiao, 2014; Zhang et al., 2015) (Table 2).
Table 2.
Characteristics of included studies in the meta-analysis
Risk of bias
The overall risk of bias of the 10 included trials was relatively low-level. Four studies did not detail how they generated random sequence (Fang et al., 2011; Guo et al., 2012; Dai et al., 2013; Cheng et al., 2014) and one study generated random sequence improperly (Zhang et al., 2015).
None of the studies provided information about allocation concealment, therefore the selection bias was unclear. Although blinding of participants and personnel was not mentioned in all included trials, the outcomes were unlikely to be affected by not implementing blinding because stem cell transplantation is a surgical treatment that cannot be performed with this approach, therefore performance bias was low risk.
Two studies did not state the blinding of outcome assessment clearly (Guo et al., 2012, 2014) and another two studies lacked blinding of outcome assessment (Xiao, 2014; Zhang et al., 2015). One study lacked follow-up information (Guo et al., 2012) and four studies made selective reporting (Fang et al., 2011; Guo et al., 2012, 2014; Xiao, 2014). No other bias was observed in these trials. Details of the methodological assessment domain are presented in Figure 2.
Figure 2.
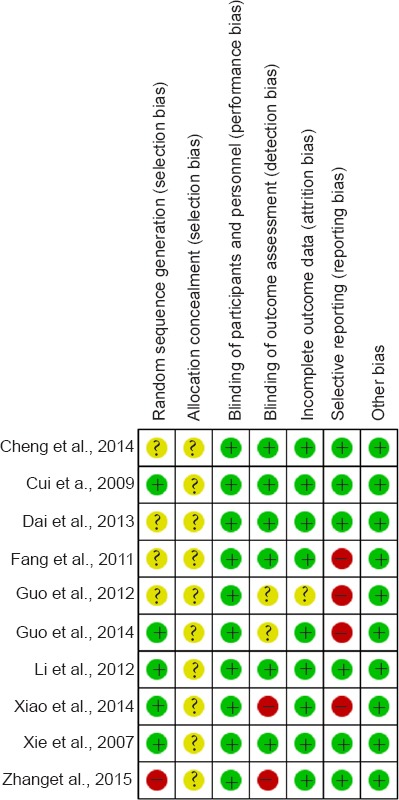
Risk of bias summary.
A review of the authors’ judgments on each risk of bias item for each included study. “+” represents “low risk”; “-” represents “high risk”; “?” represents “unclear”.
Results of meta-analysis
ASIA motor score
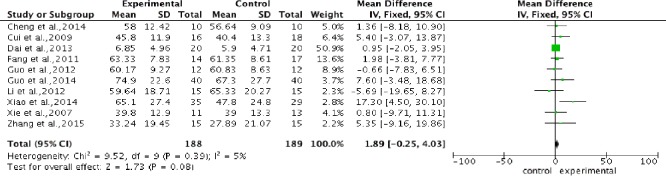
Pooled analysis of the 10 studies indicated that stem cell transplantation did not significantly improve motor score compared with rehabilitation therapy, without heterogeneity (P = 0.39, I2 = 5%). The overall change of ASIA motor score was 1.89 (95%CI: –0.25 to 4.03) (Figure 3).
Figure 3.
Comparison of American Spinal Injury Association motor score in spinal cord injury patients after stem cell transplantation and rehabilitation intervention.
AISA light touch score
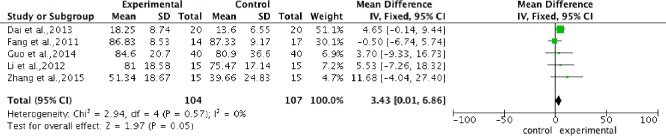
Pooled analysis of five studies (Fang et al., 2011; Li, 2012; Dai et al., 2013; Guo, 2014; Zhang et al., 2015) to assess ASIA light touch score after intervention, showed a significantly higher score in the stem cell transplantation group compared with the control group. The overall change of ASIA light touch score was 3.43 (95%CI: 0.01–6.86, P = 0.05) without heterogeneity (P = 0.57, I2 = 0%) (Figure 4).
Figure 4.
Comparison of American Spinal Injury Association light touch score in spinal cord injury patients after stem cell transplantation and rehabilitation intervention.
ASIA pinprick score
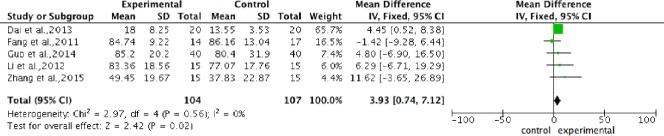
Pooled analysis of five studies (Fang et al., 2011; Li, 2012; Dai et al., 2013; Guo, 2014; Zhang et al., 2015) to assess ASIA pinprick score after intervention, showed a significantly higher score in the stem cell transplantation group compared with the control group. The overall change of ASIA pinprick score was 3.93 (95%CI: 0.74–7.12, P = 0.02) without heterogeneity (P = 0.56, I2 = 0%) (Figure 5).
Figure 5.
Comparison of American Spinal Injury Association pinprick score in spinal cord injury patients after stem cell transplantation and rehabilitation intervention.
AIS grading improvement rate
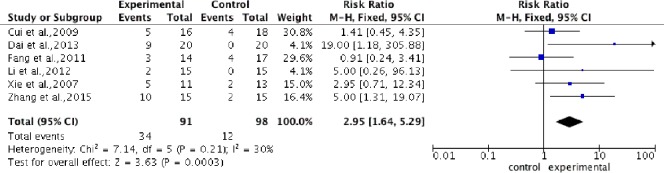
Pooled analysis of six studies (Xie et al., 2007; Cui et al., 2009; Fang et al., 2011; Li, 2012; Dai et al., 2013; Zhang et al., 2015) to assess the AIS grading improvement rate, showed a higher improvement rate in the stem cell transplantation group compared with the control group. Using a fixed-effects model, the overall change of AIS grading improvement rate was 2.95 (95%CI: 1.64–5.29, P = 0.0003) with low heterogeneity (P = 0.21, I2 = 30%) (Figure 6).
Figure 6.
Comparison of AIS grading improvement rate in spinal cord injury patients after stem cell transplantation and rehabilitation intervention.
Compared with the control group, the AIS grading improvement rate was significantly increased in the experimental group. AIS: American Spinal Injury Association impairment scale.
ADL score
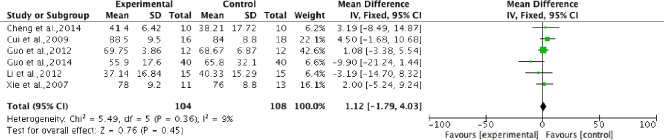
Pooled analysis of six studies (Xie et al., 2007; Cui et al., 2009; Guo et al., 2012; Li, 2012; Cheng et al., 2014; Guo, 2014) to assess the ADL score, showed an insignificantly higher score in the stem cell transplantation group compared with the control group. Using a fixed-effects model, the overall change of ADL score was 1.12 (95%CI: −1.79 to 4.03, P = 0.45) without heterogeneity (P = 0.36, I2 = 9%), indicating that there was no statistical difference in ADL score between stem cell transplantation and rehabilitation therapy (Figure 7).
Figure 7.
Comparison of activities of daily living score in spinal cord injury patients after stem cell transplantation and rehabilitation intervention.
Residual urine volume
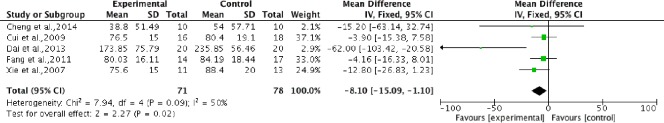
Pooled analysis of five studies (Xie et al., 2007; Cui et al., 2009; Fang et al., 2011; Dai et al., 2013; Cheng et al., 2014) to analyze residual urine volume after therapy showed that stem cell transplantation significantly reduced residual urine volume compared with rehabilitation therapy (RR = –8.1; 95%CI: –15.09 to –1.10) and moderate heterogeneity (P = 0.09, I2 = 50%) (Figure 8).
Figure 8.
Comparison of residual urine volume in spinal cord injury patients after stem cell transplantation and rehabilitation intervention.
Adverse effects
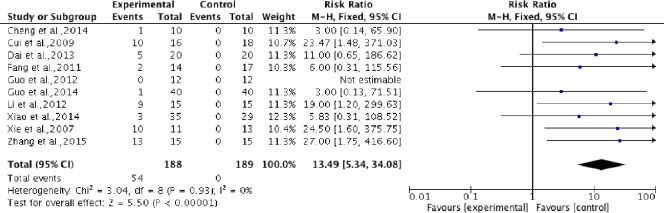
Data pooling of all ten studies was carried out to assess the relative risk of any adverse effects during treatment. Significant differences in the RR of adverse effects were identified after stem cell transplantation compared with rehabilitation therapy. The average risk ratio of these studies was 13.39 (95%CI: 5.34–34.08, P < 0.00001) without heterogeneity (P = 0.93, I2 = 0%) between studies. Generally, adverse effects caused by transplantation included fever, headache, backache, numbness, and abdominal distension, which were alleviated spontaneously or following treatment intervention. However, serious adverse effects, such as death, tumor, or immune reaction, were not observed during follow-up (Figure 9).
Figure 9.
Comparison of incidence of adverse effects in spinal cord injury patients after stem cell transplantation and rehabilitation intervention.
Publication bias
Due to the insufficient number of studies included in the meta-analysis, funnel plots were inappropriate to evaluate publication bias. Consequently, no appropriate published protocols were indicated to process the publication bias.
Discussion
Summary of evidence
Although controversy exists around the effectiveness of stem cell transplantation (Dobkin et al., 2006), the present study results show that when compared with rehabilitation therapy, stem cell transplantation can improve neurological function, especially sensory functions including light touch and pinprick, without increasing the incidence of serious adverse effects. Furthermore, stem cell transplantation offers greater reduction in residual urine volume, indicating enhanced recovery of bladder function. Although the meta-analysis shows that stem cell transplantation has no significant improvement in motor function, ADL, and some mild and temporary adverse effects, stem cell transplantation can reduce the severity of SCI. In summary, these data lend support to stem cell transplantation as a therapeutic and safe therapy to improve neurological function, bladder function, and holistic rehabilitation of patients with SCI.
As shown in the results, the present meta-analysis has concluded consistent findings with low heterogeneity, supporting the effectiveness of stem cell transplantation for functional recovery after SCI. It is reassuring that the between-study heterogeneity is low and that subgroup and sensitivity analyses are unnecessary.
Furthermore, stem cell source and transplantation method had no impact on the extent of residual urine volume, indicating that stem cell transplantation significantly enhances recovery of urination function, consistent with the outcome of a recent systematic review and meta-analysis (Kim et al., 2015) on bladder recovery using stem cell therapy after SCI. Kim et al. (2015) verified partial bladder recovery, including improvement of voiding pressure, non-voiding contraction, and residual urine, after stem cell-based therapy in SCI rats, and demonstrated that stem cell transplantation effected recovery of urination function in preclinical experiments and as a clinical therapy. This may be related to the recovery of neurological function and the low risk of urinary infection in these studies, whereby no urinary infection occurred in the SCI patients treated with stem cells. However, pooled motor score data showed that the locomotor function of patients with SCI is not improved after stem cell transplantation. This may be related to the time point of transplantation. Keirstead et al. (2005) transplanted stem cells into rats with SCI 10 months after injury, finding that transplantation in the chronic phase results in insignificant remyelination and poor locomotion improvement. It is suggested that transplantation within a therapeutic time frame of 3–4 weeks following injury is optimal for stem cell treatment of SCI (Sykova et al., 2006). In the present meta-analysis, nine out of ten studies were conducted in chronic SCI patients, making it convincing to infer that it is difficult to improve motor function in patients with chronic SCI using stem cell transplantation. Without improvements in motor function following stem cell transplantation, the ADL of SCI patients remains unimproved; meaning they cannot take care of themselves or live independently. Although some adverse effects were observed in the studies, these were minimal with temporary side effects, including fever, headache, backache, numbness, and abdominal distension, primarily caused by spinal puncture. None of the patients undergoing stem cell transplantation showed serious adverse effects such as tumor formation or death. Thus, despite the overall RR score of adverse effects being 13.49, stem cell transplantation was found to be relatively safe in the treatment of SCI. With a significant improvement in the rate of AIS grading, stem cell transplantation is effective in reducing the severity of SCI. Recently, Li et al. (2015) performed a systematic review and meta-analysis on the efficacy and safety of bone marrow-derived cell transplantation for SCI in low-quality clinical trials, including clinical control trials and randomized controlled trials. The findings reported that bone marrow-derived cell transplantation was safe and valid for patients with SCI with improvements in AIS, ASIA pinprick, ASIA light touch, and ASIA motor scores. However, the present meta-analysis study was not consistent with Li et al.'s study, possibly because of differences in the type of transplanted cells and methods of transplantation used, resulting in different effects on ASIA motor score. Therefore, it is convincing that stem cell transplantation is safe and effective for sensory function recovery after SCI, however its effect on locomotion function recovery is not clear.
Compared with other published meta-analysis studies using stem cell transplantation to treat SCI (Li, 2013, 2015; Kim et al., 2015; Yousefifard et al., 2016), the present meta-analysis has its own distinctive features. For example, more than one type of stem cell was included to evaluate the general effects and safety of stem cell transplantation in SCI patients. Another distinction is that unlike other published meta-analyses that focus on animal trials, clinical control trials, or only one recovery outcome, the present meta-analysis included more primary recovery outcomes and randomized controlled trials from which the quality of evidence is graded high in evidence-based medicine to analyze the pooled effects and safety of stem cell transplantation in SCI patients.
Limitations
There are some limitations in the present meta-analysis. Firstly, the sample size of the included trials was too small to provide sufficient evidence for the outcomes. None of the included studies had a sample size greater than 100 subjects. Secondly, because all the studies were carried out in China, it is unknown if stem cell transplantation has the same effects and safety profile in other populations. The 10 studies analyzed in this meta-analysis were identified following systematic searching of seven databases according to the inclusion criteria. The reason why no matched randomized controlled trials performed in countries other than China were found may relate to differences in ethical policies in stem cell transplantation between countries and the significantly higher incidence of SCI in China. Notably, it is comparatively stricter in countries other than in China regarding policies and ethical reviews towards stem cell transplantation research. Together, these criteria create favorable conditions for China's hospitals and experienced doctors to further medical techniques and to study stem cell transplantation through randomized controlled trials in humans with SCI. Thirdly, long-term follow-up was absent in all included studies, with a maximum of only 12 months. Teratoma formation, one of the most serious adverse effects of cell therapies, is dependent on different cell types, differentiation protocols, and transplantation of heterogeneous cell populations (Brederlau et al., 2006; Li et al., 2008), therefore it is necessary to perform long-term follow-up to ensure the long-term safety of stem cell transplantation. Fourthly, because none of these studies clearly describe allocation concealment, and four studies do not detail how random sequence was generated, some selection bias may exist in the present meta-analysis. Lastly, many factors including stem cell preparation, stem cell identification, nursing care, socioeconomic level, and the mental state of SCI patients, all of which are easily ignored, may influence outcomes of stem cell transplantation for SCI. Further trials with reasonable random sequence generation, adequate allocation concealment, and low risk of reporting bias, are critical to assess and clarify the effects and safety of stem cell transplantation for SCI. Additionally, more multicentric and large-sample randomized controlled trials of stem cell transplantation need to be performed to provide more medical evidence-based proofs. Methods of stem cell transplantation should also be improved to reduce the incidence of side effects related to transplantation.
Conclusions
Stem cell transplantation was determined to be an efficient and safe treatment for SCI and simultaneously improved sensory and bladder functions. Although associated minor and temporary adverse effects were observed with transplanted stem cells, spinal cord repair and axon remyelination were apparent. It is necessary to combine stem cell transplantation with other therapies, such as rehabilitation exercise, to promote locomotion recovery. More randomized controlled trials with larger sample sizes and longer follow-up times are needed to further validate the effectiveness of stem cell transplantation in the treatment of SCI. Furthermore, transplantation method refinements should be made to improve the effects of stem cell transplantation.
Acknowledgments
We are very grateful to Rui Xia for providing guidance for the meta-analysis.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81273775.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Armijo-Olivo S, Bn CRSR, Frcpc NAH, Biondo PD, Rn GGC. Assessment of study quality for systematic reviews: a comparison of the cochrane collaboration risk of bias tool and the effective public health practice project quality assessment tool: methodological research. J Eval Clin Pract. 2012;18:12–18. doi: 10.1111/j.1365-2753.2010.01516.x. [DOI] [PubMed] [Google Scholar]
- Baaj A, Uribe J, Nichols T, Theodore N, Crawford N, Sonntag V, Vale F. Health care burden of cervical spine fractures in the United States: analysis of a nationwide database over a 10-year period. J Neurosurg Spine. 2010;13:61–66. doi: 10.3171/2010.3.SPINE09530. [DOI] [PubMed] [Google Scholar]
- Bakshi A, Barshinger AL, Swanger SA, Madhavani V, Shumsky JS, Neuhuber B, Fischer I. Lumbar puncture delivery of bone marrow stromal cells in spinal cord contusion: a novel method for minimally invasive cell transplantation. J Neurotrauma. 2006;23:55–65. doi: 10.1089/neu.2006.23.55. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- Becker D, McDonald JW., 3rd Approaches to repairing the damaged spinal cord: overview. Handb Clin Neurol. 2012;109:445–461. doi: 10.1016/B978-0-444-52137-8.00028-0. [DOI] [PubMed] [Google Scholar]
- Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, DeVries WH, Shields CB, Magnuson DS, Xu XM, Kim DH, Whittemore SR. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 2010;30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Chen Y, DeVivo M. Lifetime direct costs after spinal cord injury. Top Spinal Cord Inj Rehabil. 2011;16:10–16. [Google Scholar]
- Cheng H, Liu X, Hua R, Dai G, Wang X, Gao J, An Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253–260. doi: 10.1186/s12967-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui GX, Song CZ, Li YS, Yue SW. Clinical study of autologous marrow mononuclear cells transplantation in patients with spinal cord injury. Zhongguo Kangfu Yixue Zazhi. 2009;4:309–312. [Google Scholar]
- Dai G, Liu X, Zhang Z, Yang Z, Dai Y, Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. doi: 10.1016/j.brainres.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Dobkin BH, Curt A, Guest J. Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair. 2006;20:5–13. doi: 10.1177/1545968305284675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erceg S, Ronaghi M, Oria M, Rosello MG, Arago MA, Lopez MG, Radojevic I, Moreno-Manzano V, Rodriguez-Jimenez FJ, Bhattacharya SS, Cordoba J, Stojkovic M. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010;28:1541–1549. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang ML, Wang MX, Wang YQ, Wei XJ, Wang JQ. Autologous bone marrow stromal cells derived stem cells in the treatment of spinal cord injury. J Qiqihar Univ Med. 2011;32:2064–2066. [Google Scholar]
- Genovese T, Cuzzocrea S. Role of free radicals and poly(ADP-ribose)polymerase-1 in the development of spinal cord injury: new potential therapeutic targets. Curr Med Chem. 2008;15:477–487. doi: 10.2174/092986708783503177. [DOI] [PubMed] [Google Scholar]
- Guo GH, Shen LF, Li Z. Clinical study of umbilical cord blood mesenchymal stem cells transplantation on spinal cord injury. Chin J Pract Med. 2012;39:58–60. [Google Scholar]
- Guo ZS, Qin BY, Dai RQ, Shao HZ, Cheng JJ, Zhang HF, Liu WQ. Bone marrow mesenchymal stem cells in the treatment of spinal cord injury. Zhonghua Shiyan Waike Zazhi. 2014;31:2605–2607. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun JK, Kim HW. Clinical and experimental advances in regeneration of spinal cord injury. J Tissue Eng. 2010;2010:650857. doi: 10.4061/2010/650857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide C, Kanekiyo K. Points regarding cell transplantation for the treatment of spinal cord injury. Neural Regen Res. 2016;11:1046–1049. doi: 10.4103/1673-5374.187021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Kim SH. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma. 2010;27:131–138. doi: 10.1089/neu.2008.0818. [DOI] [PubMed] [Google Scholar]
- Kim JH, Shim SR, Doo SW, Yang WJ, Yoo BW, Kim JM, Ko YM, Song ES, Lim IS, Lee HJ, Song YS. Bladder recovery by stem cell based cell therapy in the bladder dysfunction induced by spinal cord injury: systematic review and meta-analysis. PLoS One. 2015;10:e0113491. doi: 10.1371/journal.pone.0113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey M. International standards for neurological classification of spinal cord injury (revised 2011) J Spinal Cord Med. 2011;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AS, Harvey AR, Plant GW, Hodgetts SI. Systematic review of induced pluripotent stem cell technology as a potential clinical therapy for spinal cord injury. Cell Transplant. 2013;22:571–617. doi: 10.3727/096368912X655208. [DOI] [PubMed] [Google Scholar]
- Li M. The clinical study of stem cells transplantation for treatment of spinal cord injury. Kuming: Kuming Medical University; 2012. [Google Scholar]
- Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31:146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Li XC, Zhong CF, Deng GB, Liang RW, Huang CM. Efficacy and safety of bone marrow-derived cell transplantation for spinal cord injury: a systematic review and meta-analysis of clinical trials. Clin Transplant. 2015;29:786–795. doi: 10.1111/ctr.12580. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and Elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maikos JT, Shreiber DI. Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J Neurotrauma. 2007;24:492–507. doi: 10.1089/neu.2006.0149. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- Min SH, Lee SH, Shim H, Park JS, Lee YI, Kim HW, Hyun JK. Development of complete thoracic spinal cord transection model in rats for delayed transplantation of stem cells. Spine. 2011;36:E155–163. doi: 10.1097/BRS.0b013e3181d8b92a. [DOI] [PubMed] [Google Scholar]
- Moreno-Manzano V, Rodriguez-Jimenez FJ, Garcia-Rosello M, Lainez S, Erceg S, Calvo MT, Ronaghi M, Lloret M, Planells-Cases R, Sanchez-Puelles JM, Stojkovic M. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells. 2009;27:733–743. doi: 10.1002/stem.24. [DOI] [PubMed] [Google Scholar]
- Mothe AJ, Tator CH. Transplanted neural stem/progenitor cells generate myelinating oligodendrocytes and Schwann cells in spinal cord demyelination and dysmyelination. Exp Neurol. 2008;213:176–190. doi: 10.1016/j.expneurol.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Nowrouzi B, Assan-Lebbe A, Sharma B, Casole J, Nowrouzi-Kia B. Spinal cord injury: a review of the most-cited publications. Eur Spine J. 2016:1–2. doi: 10.1007/s00586-016-4669-z. [DOI] [PubMed] [Google Scholar]
- Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kataoka K, Chou H, Ishikawa N, Matsumoto N, Iwashita Y. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187:266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Pannek J, Pannek-Rademacher S, Jus MS, Krebs J. Homeopathic prophylaxis for recurrent urinary tract infections following spinal cord injury: study protocol for a randomized controlled trial. Asia Pac J Clin Trials Nerv Syst Dis. 2016;1:191–195. [Google Scholar]
- Parr AM, Kulbatski I, Zahir T, Wang X, Yue C, Keating A, Tator CH. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience. 2008;155:760–770. doi: 10.1016/j.neuroscience.2008.05.042. [DOI] [PubMed] [Google Scholar]
- Post MW, van Leeuwen CM. Psychosocial issues in spinal cord injury: a review. Spinal Cord. 2012;50:382–389. doi: 10.1038/sc.2011.182. [DOI] [PubMed] [Google Scholar]
- Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- Ruff CA, Wilcox JT, Fehlings MG. Cell-based transplantation strategies to promote plasticity following spinal cord injury. Exp Neurol. 2012;235:78–90. doi: 10.1016/j.expneurol.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Sandner B, Prang P, Rivera FJ, Aigner L, Blesch A, Weidner N. Neural stem cells for spinal cord repair. Cell Tissue Res. 2012;349:349–362. doi: 10.1007/s00441-012-1363-2. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Radtke C, Tan AM, Zhao P, Hamada H, Houkin K, Honmou O, Kocsis JD. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci. 2009;29:14932–14941. doi: 10.1523/JNEUROSCI.2769-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova E, Homola A, Mazanec R, Lachmann H, Konradova SL, Kobylka P, Padr R, Neuwirth J, Komrska V, Vavra V, Stulik J, Bojar M. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15:675–687. doi: 10.3727/000000006783464381. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Natsume A, Wakabayashi T, Aoshima C, Shimato S, Ito M, Ishii J, Maeda Y, Hara M, Kim SU. Intravenously transplanted human neural stem cells migrate to the injured spinal cord in adult mice in an SDF-1- and HGF-dependent manner. Neurosci Lett. 2007;426:69–74. doi: 10.1016/j.neulet.2007.08.048. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Ung RV, Lapointe NP, Rouleau P, Guertin PA. Non-assisted treadmill training does not improve motor recovery and body composition in spinal cord-transected mice. Spinal cord. 2010;48:750–755. doi: 10.1038/sc.2010.19. [DOI] [PubMed] [Google Scholar]
- Vaquero J, Zurita M, Oya S, Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: systemic or local administration? Neurosci Lett. 2006;398:129–134. doi: 10.1016/j.neulet.2005.12.072. [DOI] [PubMed] [Google Scholar]
- Volarevic V, Erceg S, Bhattacharya SS, Stojkovic P, Horner P, Stojkovic M. Stem cell-based therapy for spinal cord injury. Cell Transplant. 2013;22:1309–1323. doi: 10.3727/096368912X657260. [DOI] [PubMed] [Google Scholar]
- Wu MF, Zhang SQ, Gu R, Liu JB, Li Y, Zhu QS. Transplantation of erythropoietin gene-modified neural stem cells improves the repair of injured spinal cord. Neural Regen Res. 2015;10:1483–1490. doi: 10.4103/1673-5374.165521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- Xiao YL, Li ZM, Zhu JX, Guo CJ, Geng FY, Zhang ZD, Zhong ZL, Han FB. Efficacy observation of autologous bone marrow-derived mesenchymal stem cell therapy on early spinal cord injury. Zhonghua Shengwu Yixue Gongcheng Zazhi. 2014;20:7–11. [Google Scholar]
- Xie ZW, Cui GX, Li YZ, Li BW, Zhu SW, Song CZ, Shi Q, Hou HS, Shen BJ. Curative effect of autologous mesenchymal stem cell transplantation on spinal cord injury. J Clin Rehabil Tissue Eng Res. 2007;11:1277–1279. [Google Scholar]
- Yousefifard M, Rahimi-Movaghar V, Nasirinezhad F, Baikpour M, Safari S, Saadat S, Jafari AM, Asady H, Tousi SMTR, Hosseini M. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. J Neurosci. 2016;322:377–397. doi: 10.1016/j.neuroscience.2016.02.034. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhu XQ, Zhang DC. Transplantation of bone marrow mesenchymal stem cells overexpressing Shootin1 for treatment of spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:7507–7517. [Google Scholar]
- Zhang Z, Dai GH, Liu XB, Wang XD, An YH. Umbilical cord mesenchymal stem cell transplantation for spinal cord injury. Zhonghua Shiyong Zhenduan yu Zhiliao Zazhi. 2015;29:478–480. [Google Scholar]