Figure 1.
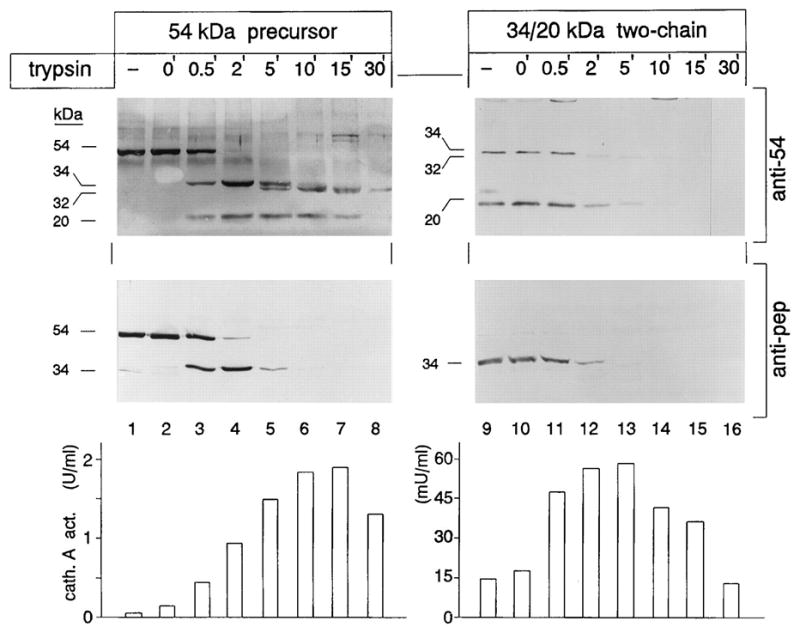
Limited proteolysis with trypsin of 54-kDa precursor and 34- and 20-kDa reconstituted two-chain protein. Aliquots of medium concentrates containing the 54-kDa precursor and 34- and 20-kDa associated protein were incubated at 37 °C with 1 mg of trypsin in the presence of bovine serum albumin (1 mg/ml) for the indicated periods of time. Reactions were stopped with 3 mg of trypsin inhibitor. Samples in lanes 1 and 9 were untreated. At time 0 (lanes 2 and 10), the samples were treated with trypsin inhibitor prior to the addition of trypsin. A portion of each sample was separated by SDS-polyacrylamide gel electrophoresis, followed by electroblotting and immunostaining with anti-54 and anti-pep antibodies. Cathepsin A activity toward the acylated dipeptide benzyloxycarbonyl-phenylalanyl-alanine was measured in each aliquot. One milliunit (mU) of activity is defined as the enzyme activity that releases one nanomole of alanine/minute. Adapted from Bonten et al JBC 1995, 30 with permission of JBC.
