Abstract
A prognostic biomarker for IgA nephropathy (IgAN) recurrence after renal transplant is lacking. We followed 96 consecutive first renal transplant recipients with native kidney IgAN (79 men; 92 deceased donors; mean age =48.1 years) on calcineurin inhibitor–based immunosuppression over 10 years for death, allograft failure, and clinicopathologic recurrence (CPR; clinically evident and biopsy-proven). Using time-dependent Cox regression analysis and receiver operating characteristic curves, we assessed prognostic significance of levels of galactose-deficient IgA1 (Gd-IgA1; autoantigen) and Gd-IgA1–specific IgG and IgA autoantibodies in serum obtained at time of transplant or native-kidney IgAN diagnosis (30 patients only). Overall, 13 patients died, 34 kidneys failed (17 due to CPR), and 34 patients developed CPR after a mean interval of 5.8 years. Compared with healthy controls (n=30), patients had significantly elevated serum Gd-IgA1 levels at diagnosis and transplant, but levels did not associate with any outcome. Patients also had significantly elevated levels of normalized (but not total) serum Gd-IgA1–specific IgG autoantibodies at diagnosis and transplant, and the level at transplant associated with higher risk of CPR (relative risk, 2.68; 95% confidence interval, 1.26 to 5.71; P=0.01; area under the receiver operating characteristic curve, 0.62; 95% confidence interval, 0.51 to 0.74; P=0.05). Normalized Gd-IgA1–specific IgG autoantibody level remained an independent risk factor for CPR in multivariate analysis. Serum Gd-IgA1–specific IgA autoantibody level did not change between diagnosis and transplant or predict outcome. This study emphasizes post-transplant prognostic value of normalized serum IgG antiglycan autoantibody level in patients with IgAN.
Keywords: IgA nephropathy, renal transplantation, IgA deposition, transplant outcomes, recurrent disease
IgA nephropathy (IgAN) was first described in 19681 and remains the most common type of primary glomerular disease, frequently leading to ESRD2 with the need of chronic dialysis or renal transplantation. The prevalence for ESRD is high: between 30% and 60% of the affected patients in many countries.2,3 The cumulative incidence at 10 years from onset in the Saint Etienne cohort3 was about 15%.
IgAN is considered a systemic disease because of its frequent recurrence after transplantation4 and clearance of IgA mesangial deposits from donor kidneys with subclinical disease within a few months after engraftment into recipients with non-IgAN renal disease.5,6 The pathophysiology of native-kidney IgAN has been clarified in recent years,7,8 and the mechanisms of disease are now considered to be of an autoimmune nature. Galactose-deficient IgA1 (Gd-IgA1), with a reduced number of galactose molecules attached to the hinge region O-glycans, is the major autoantigen; IgG specific for Gd-IgA1 (IgG-autoAb) or IgA specific for Gd-IgA1 (IgA-autoAb) is the autoantibody.9 We10 and others have shown a correlation between serum levels of autoantigen11 and autoantibodies and the risk of progression of the native kidney disease to ESRD.12
Recurrence of IgAN after renal transplantation13–17 is frequent and clearly the incidence increases with the duration of follow-up; the 10-year cumulative recurrence rate has been about 50% at many centers,14,16 and in our experience, it was 36%.18 For renal transplant recipients, the outcome after transplantation can be quite varied, as it is for patients with other forms of native disease. Outcomes after transplantation include death (due mainly to cardiovascular disease, cancer, or infection), allograft failure due to cellular or antibody-mediated rejection, and, sometimes, severe infection with specific morbidity and mortality; in addition, patients with IgAN face the risk of recurrent disease that may adversely affect long-term allograft function and, sometimes, lead to allograft loss.15
In this paper, we focused on clinicopathologic recurrence (CPR) clinically evident and proven by allograft biopsy. The goals of this study with patients with IgAN were to assess outcomes after first renal transplantation (death, allograft failure [all causes], and recurrence of IgAN) and to use stored serum samples to measure levels of Gd-IgA1 and IgG and IgA autoantibodies specific for Gd-IgA1 and correlate these data with the long-term outcomes.
Results
Characteristics of Transplant Recipients at Baseline
The cohort included 79 (82.3%) men and 17 women who had received their first kidney allograft for ESRD due to biopsy-proven native kidney IgAN. At the time of engraftment, their mean age was 48.1 years (SD=14.2); mean age of the donors was 42.0 years (92 were deceased donors) (Supplemental Table 1).
Over a mean follow-up of 12.4 years (SD=6.1), 13 patients had died (prevalence of 13.5%), 34 allografts had failed (35.4%, with 17 due to CPR, 13 due to rejection, and four due to various other causes). Thirty-four recipients developed CPR of IgAN (prevalence rate of 35.4% for all transplant recipients), which progressed to allograft loss in 17 recipients. The first composite event (death, allograft failure, or CPR) was reached in 58 recipients: death (11), allograft loss (13), and CPR (34) for an overall prevalence of 60.4%).
The deaths occurred at a mean post-transplantation interval of 10.8 years (SD=4.1), with a median of 10.1 years (range =5.4–18.6). The allograft failures (all in stage 5 CKD and needing dialysis) occurred at a mean interval of 10.4 years (SD=5.9) and a median interval of 9.1 years (range =0.02–25.5). The CPR events started earlier at a mean interval of 5.8 years (SD=4.3) with a median of 5.3 years (range =0.4–18.9), with 75% of these events occurring within 8.79 years and 90% occurring within 9.94 years. The 17 allograft losses due to CPR occurred after a mean post-transplant interval of 11.2 years (SD=6.3), and the mean interval between the diagnosis of CPR and allograft loss due to CPR was 5.7 years (SD=4.8).
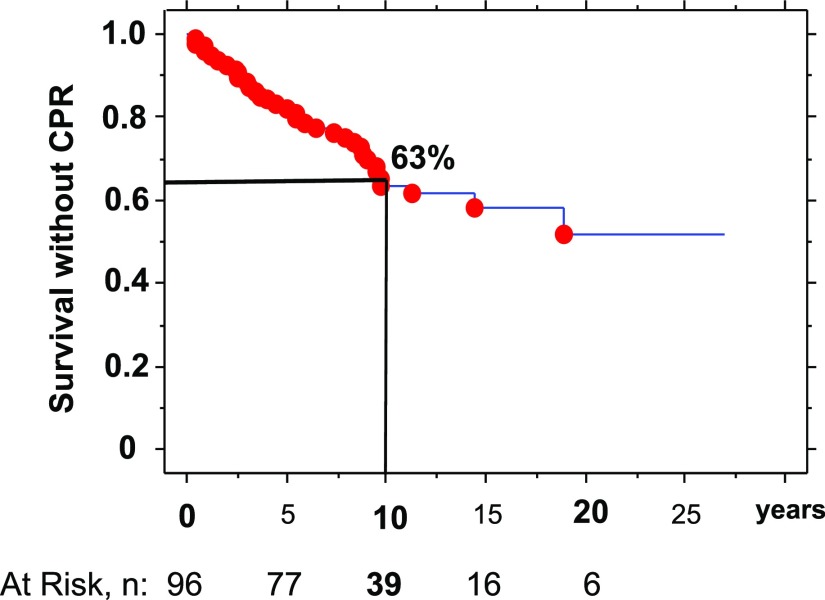
The 10-year cumulative incidence rates, derived from the Kaplan–Meier survival analysis, were 7.6% for death (at risk, n=56), 22.8% for allograft failure alone (at risk, n=56), 28.7% for combined death and allograft loss (allograft survival; at risk, n=56), and 12.3% for allograft loss due to CPR (at risk, n=56) (Table 1). In addition, the 10-year cumulative incidence rate was 36.5% for CPR (at risk, n=39) (Figure 1).
Table 1.
Comparisons of 10-year survival rate and 10-year incidence rate according to the type of event
| Type of Event | 10-yr No. of Event | 10-yr Cum Survival Rate without Event, % | 10-yr Cum Incidence Rate of Event, % | 10-yr No. of Patients at Risk |
|---|---|---|---|---|
| Death | 6 | 92 | 8 | 56 |
| Return to dialysis | 20 | 77 | 23 | 56 |
| Either | 26 | 71 | 29 | 56 |
| CPR | 31 | 63 | 37 | 39 |
| Early CPR | 26 | 64 | 36 | 30 |
| Composite | 44 | 52 | 48 | 39 |
| Allograft loss due to CPR | 10 | 88 | 12 | 56 |
Cum, cumulative; early CPR, 75% of CPR events occurring before 8.79 years after transplantation (either death/return to dialysis or composite [death, return to dialysis, or CPR]).
Figure 1.
Kaplan–Meier survival curve without CPR event. The curve depicts the cumulative survival according to time after transplantation. The 10-year point gave 63% survival and a 37% cumulative incidence rate for CPR, with 39 recipients at risk.
Serum Biomarkers (IgA, Autoantigen, IgG, IgG Autoantibody, and IgA Autoantibody) in Healthy Controls and Patients with IgAN at the Time of Native Kidney Diagnostic Biopsy and the Time of First Kidney Transplantation
Archived serum samples collected at the time of diagnostic native kidney biopsy were available for 30 patients with IgAN. The mean interval between diagnosis and first kidney transplantation for these patients was 7.07 years (SD=6.46). The mean serum total Gd-IgA1 (autoantigen) level was elevated at the time of native disease diagnosis in 30 patients with IgAN (P=0.001) compared with healthy controls along with the mean level on the day of transplantation for all 96 recipients (P=0.001). The levels in the patients did not differ at these two time points (Table 2). Mean serum IgA levels were also elevated at both time points. Mean normalized (expressed for a normalized amount of IgG at 0.5 μg) IgG-autoAb was elevated compared with that in controls at the time of IgAN diagnosis (P=0.001) and transplantation (P=0.001) but with a significant decrease in the interval between native disease diagnosis and renal transplantation (P=0.001 by unpaired t test and P=0.001 by paired t test). For the total IgA-autoAb mean level, we observed only a slight elevation at the time of native kidney diagnosis (P=0.03).
Table 2.
Biomarker values in healthy controls and patients with IgAN at the time of diagnosis by native kidney biopsy and the time of renal transplantation
| Serum Biomarker | Healthy Controls | IgAN | Unpaired t Test | |
|---|---|---|---|---|
| Diagnosis (Dg) | Transplantation (Tx) | |||
| No. | 30 | 30 | 96 | |
| Serum biomarkera | At Tx versus at Dg | |||
| IgA, mg/ml | 2.20 (0.76)/2.09 | 3.16 (1.26)/2.90 | 2.99 (1.35)/2.68 | NS |
| Unpaired t test versus controls | <0.001 (t=−3.61) | 0.003 (t=3.07) | ||
| Norm Gd-IgA, % HAA | 31.3 (9.1)/29.5 | 35.6 (14.5)/36.1 | 36.1 (12.6)/33.6 | NS |
| Unpaired t test versus controls | NS | 0.05 (t=1.95) | ||
| Total Gd-IgA, U/ml | 68.2 (26.0)/65.4 | 111.1 (61.4)/97.9 | 105.4 (54.2)/99.9 | NS |
| Unpaired t test versus controls | <0.001 (t=−3.52) | <0.001 (t=3.62) | ||
| IgG, mg/ml | 11.01 (2.64)/10.54 | 8.35 (2.19)/8.29 | 10.16 (4.53)/9.43 | 0.04 (t=2.11)b |
| Unpaired t test versus controls | <0.001 (t=4.24) | NS | ||
| Norm IgG-autoAb, OD | 1.172 (0.242)/1.195 | 1.795 (0.535)/1.617 | 1.465 (0.447)/1.498 | 0.001 (t=−3.37)c |
| Unpaired t test versus controls | <0.001 (t=−3.87) | <0.001 (t=3.43) | ||
| Total IgG-autoAb, U/ml | 25.60 (7.28)/24.34 | 30.36 (12.59)/29.33 | 30.55 (17.90)/27.04 | |
| Unpaired t test versus controls | NS (0.08) | NS (0.14) | ||
| Norm IgA-autoAb, OD | 0.549 (0.234)/0.458 | 0.715 (0.707)/0.361 | 0.611 (0.580)/0.462 | |
| Unpaired t test versus controls | NS | NS | ||
| Total IgA-autoAb, U/ml | 1.29 (0.91)/0.93 | 2.31 (2.41)/1.51 | 2.00 (2.28)/1.22 | |
| Unpaired t test versus controls | 0.03 (t=−2.16) | NS (0.10) | ||
Norm, normalized; HAA, Helix aspersa agglutinin;
Laboratory data shown as mean (SD)/median. Total Gd-IgA1 was calculated by multiplying HAA binding in percentage by the IgA concentration and is expressed in units per milliliter. Total IgG-autoAb was calculated by multiplying OD per 0.5 μg by the IgG concentration ×2 and is expressed in units per milliliter. Total IgA-autoAb was calculated by multiplying OD per 1 μg by the IgA concentration and is expressed in units per milliliter.
NS by paired t test.
P=0.001 (t=−4.36) by paired t test (30 at both date of diagnosis by native kidney biopsy and date of renal transplantation).
Table 3 shows the comparison of the serum biomarker levels in the 34 recipients who exhibited recurrence of IgAN (CPR positive) versus the other 62 patients (CPR negative). Among the biomarkers, only the mean normalized serum IgG-autoAb level was elevated in recipients with recurrent IgAN (P<0.01). Recurrence of disease was clearly a time-dependent event. We set an upper limit corresponding to the onset of 75% of the CPR events: 26 events started before 8.79 years post-transplantation. Eleven patients died without recurrence, and eight CPR events occurred after this interval; these 19 recipients were excluded from this analysis. We then compared the 26 early CPR–positive recipients with the 51 early CPR–negative recipients. In this restricted analysis, mean serum IgG-autoAb levels (both normalized, P=0.02 and total, P=0.04) and mean normalized serum IgA-autoAb level (P=0.02) were significantly elevated before transplantation.
Table 3.
Serum biomarkers values according to CPR event or not (no time limit) and early CPR event after renal transplantation
| Event | CPR | Unpaired t Test | Early CPR | Unpaired | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| No. | 34 | 62 | 26 | 51 | ||
| Serum biomarkera | ||||||
| IgA, mg/ml | 3.19 (1.20) | 2.04 (1.43) | NS | 3.30 (1.32) | 2.94 (1.38) | NS |
| Norm Gd-IgA1, % | 35.5 (10.8) | 36.5 (13.5) | NS | 34.2 (9.6) | 36.0 (12.3) | NS |
| Total Gd-IgA1, U/ml | 110.6 (46.5) | 102.6 (58.2) | NS | 110.1 (49.3) | 101.3 (50.7) | NS |
| IgG, mg/ml | 10.93 (5.10) | 9.73 (4.17) | NS | 11.14 (5.57) | 9.75 (3.91) | NS |
| Norm IgG-autoAb, OD | 1.570 (0.430) | 1.400 (0.450) | <0.01; t=−1.80 | 1.600 (0.460) | 1.370 (0.380) | 0.02; t=−2.35 |
| Total IgG-autoAb, U/ml | 34.2 (17.7) | 28.5 (17.9) | NS (0.14) | 35.8 (19.5) | 27.5 (14.3) | 0.04; t=−2.12 |
| Norm IgA-autoAb, OD | 0.670 (0.630) | 0.580 (0.550) | NS | 0.740 (0.700) | 0.480 (0.270) | 0.02; t=−2.41 |
| Total IgA-autoAb, U/ml | 2.2 (2.2) | 1.9 (2.4) | NS | 2.5 (2.4) | 1.6 (1.6) | NS (0.06) |
Early CPR, CPR events occurring before 8.79 years after transplantation and corresponding to the 75th percentile of events with exclusion of eight recipients with later CPR and 11 recipients who died without previous recurrence; norm, normalized.
Laboratory data shown as mean (SD).
Prediction Value of Serum Biomarkers for the Different Outcomes Evaluated by the Time-Dependent Cox Regression Analyses
Prediction of Death, Return to Dialysis, and Allograft Failure Including Death
Cox regression analysis with eight serum biomarkers levels showed no significant influence by IgA, normalized Gd-IgA1, total Gd-IgA1, IgG, normalized IgG-autoAb, total IgG-autoAb, normalized IgA-autoAb, and total IgA-autoAb.
Prediction of CPR Event
Thirty-four recipients with the event were included in this analysis, and 62 recipients without the event were censored (Table 4). Overall, serum level of normalized IgG-autoAb predicted recurrence of IgAN after transplantation: relative risk (RR), 12.21; 95% confidence interval (95% CI), 1.15 to 129.79; P=0.04; β/SEM=+2.07.
Table 4.
Cox regression analyses (univariate and multivariate) assessments of biomarkers for prediction of 34 CPR events
| Serum Biomarker | RR (95% CI) | P Value | β/SEM |
|---|---|---|---|
| Univariate | |||
| IgA, mg/ml | 1.25 (0.99 to 1.57) | NS (0.06) | +1.87 |
| Norm Gd-IgA1, % | NS | ||
| Total Gd-IgA1, U/ml | NS | ||
| IgG, mg/ml | 1.08 (1.00 to 1.16) | 0.05 | +1.97 |
| Norm IgG-autoAb, OD | 2.68 (1.26 to 5.71) | 0.01 | +2.55 |
| Total IgG-autoAb, U/ml | 1.02 (1.00 to 1.04) | 0.01 | +2.49 |
| Norm IgA-autoAb, OD | NS | ||
| Total IgA-autoAb, U/ml | NS | ||
| Multivariate | |||
| IgG, mg/ml | NS | ||
| Norm IgG-autoAb, OD | 12.21 (1.15 to 129.79) | 0.04 | +2.07 |
| Total IgG-autoAb, U/ml | NS |
Only variables significant in the univariate analysis were included in this multivariate analysis. RR is also called hazard ratio; β/SEM indicates the weight of the covariate with increasing (+) and decreasing (−) the risk. Gd-IgA1 is the major autoantigen. Total (units per milliliter) is obtained by multiplying Norm value by the serum concentration of IgG or IgA as appropriate. Norm, normalized.
Prediction of Early CPR Event (<8.79 Years after Transplantation)
At that time point, 26 recipients had presented a CPR event (76.5%), and 51 recipients without the event were censored (Table 5). The serum autoantigen level had no predictive value, whereas levels of IgG and IgA-autoAb (normalized and total) were each significant predictors. Curiously, multivariate analysis identified only total IgG-autoAb as a predictor (P=0.06).
Table 5.
Cox regression analyses (univariate and multivariate) assessments of biomarkers for prediction of early CPR events
| Serum Biomarker | RR (95% CI) | P Value | β/SEM |
|---|---|---|---|
| Univariate | |||
| IgA, mg/ml | NS | ||
| Norm Gd-IgA1, % | NS | ||
| Total Gd-IgA1, U/ml | NS | ||
| IgG, mg/ml | 1.07 (0.99 to 1.16) | NS (0.10) | +1.67 |
| Norm IgG-autoAb, OD | 3.47 (1.38 to 8.73) | <0.01 | +2.64 |
| Total IgG-autoAb, U/ml | 1.03 (1.01 to 1.05) | 0.01 | +2.59 |
| Norm IgA-autoAb, OD | 2.20 (1.31 to 3.67) | 0.003 | +3.00 |
| Total IgA-autoAb, U/ml | 1.29 (1.09 to 1.52) | 0.003 | +2.94 |
| Multivariate | |||
| Norm IgG-autoAb, OD | NS | ||
| Total IgG-autoAb, U/ml | 1.03 (1.00 to 1.05) | 0.06 | +1.84 |
| Norm IgA-autoAb, OD | NS | ||
| Total IgA-autoAb, U/ml | NS |
The four variables significant in the univariate were used for multivariate analysis. β/SEM indicates the weight of the covariate with increasing (+) and decreasing (−) the risk. Early CPR indicates CPR occurring before 8.79 years after transplantation and corresponding to the 75th percentile of events (n=26) with exclusion of eight patients who developed CPR later and 11 patients dying without previous CPR. Gd-IgA1 is the major autoantigen. Norm, normalized.
Prediction of Allograft Loss Due to CPR
The global analysis (17 patients with event versus 79 patients censored) failed to show that any of these eight biomarkers predicted allograft loss due to CPR (Table 6). The analysis restricted to the causes of allograft losses (17 due to CPR versus 17 due to other causes) showed that only the serum level of total IgA-autoAb predicted a CPR-associated loss by Cox univariate analysis: RR, 1.41; 95% CI, 1.10 to 1.81; P<0.01; β/SEM=+2.67.
Table 6.
Cox regression analysis (univariate) assessment of biomarkers for prediction of allograft loss due to CPR versus other causes
| Serum Biomarker | RR (95% CI) | P Value | β/SEM |
|---|---|---|---|
| Univariate | |||
| IgA, mg/ml | NS (0.12) | ||
| Norm Gd-IgA1, % | NS | ||
| Total Gd-IgA1,U/ml | NS | ||
| IgG, mg/ml | NS | ||
| Norm IgG-autoAb, OD | NS | ||
| Total IgG-autoAb, U/ml | NS | ||
| Norm IgA-autoAb, OD | 2.34 (0.94 to 5.84) | NS (0.07) | +1.82 |
| Total IgA-autoAb, U/ml | 1.41 (1.10 to 1.81) | <0.01 | +2.67 |
Thirty-four recipients lost their allograft: 17 due to CPR and 17 due to other causes. β/SEM indicates the weight of the covariate with increasing (+) and decreasing (−) the risk. Gd-IgA1 is the major autoantigen. Norm, normalized.
Predictive Value of the Eight Serum Biomarkers for the Different Outcomes Evaluated by Receiver Operating Characteristic Curves with C Statistics
Prediction of CPR
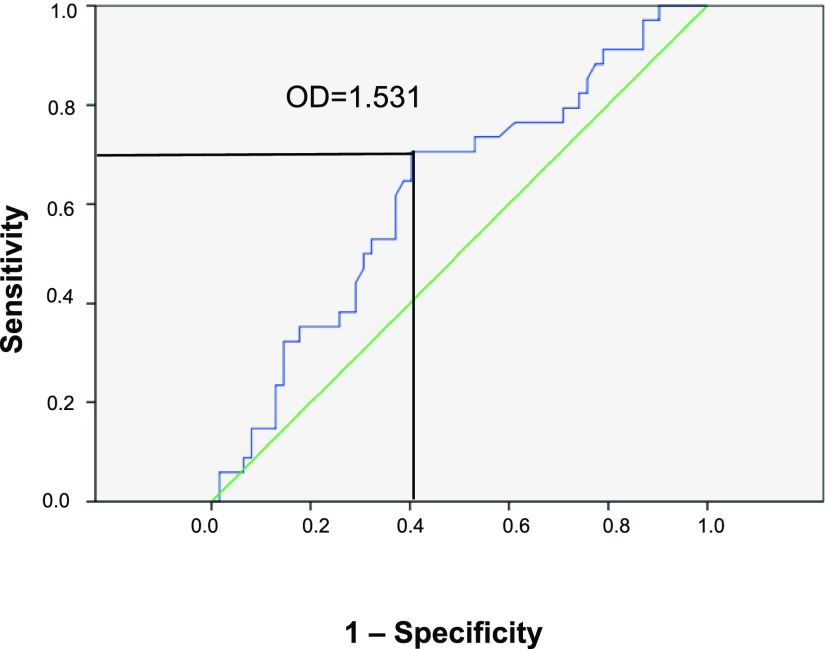
There were 34 patients with the event, and 62 patients without the event were censored. Only normalized serum IgG-autoAb level was predictive: area under the curve =0.622; 95% CI, 0.51 to 0.74; P=0.05; the best point was 1.531 (OD units), with Se=68% and Sp=63% (Figure 2). Using this threshold value of 1.531 for normalized IgG-autoAb, we found 50 normal values versus 46 high values. The contingency table for positive CPR event and high normalized IgG-autoAb had a significant chi-squared value of 5.95 with exact (Fisher) P=0.02. The Se was 22/34=0.65, and the Sp was 38/62=0.61. The positive predictive value was low (22/46=0.48), but the negative predictive value was high (38/50=0.76); the ratio [positive predictive value/(1− negative predictive value)] was 1.99. These low prediction values may reflect the difficulty of fixing a cutoff time for CPR occurrence and, also, that factors other than IgG-autoAb play a significant role in the development of CPR. For serum total IgG-autoAb in units per milliliter, the prediction was not significant (P=0.07).
Figure 2.
Receiver operator curve between CPR event and the serum level of normalized IgG autoantibody. The area under the curve is 0.622 (95% CI, 0.51 to 0.74) with P=0.05; the best compromise point is for OD value of 1.531, with sensitivity of 68% and specificity of 63%.
Prediction of Early CPR Events (<8.79 Years after Transplantation)
The 26 patients with the event and the 51 patients without the event were censored. Only serum level of normalized IgG-autoAb was predictive: area under the curve =0.648; 95% CI, 0.52 to 0.78; P=0.03. The best point was 1.519 (OD units), with Se=65% and Sp=61%.
Prediction of Allograft Loss Due to CPR
The 17 patients with this event and the 79 patients without the event were censored. None of the biomarkers was predictive of allograft loss in a global analysis or an analysis restricted to the causes of allograft loss (17 due to CPR versus 17 due to other causes).
Other Factors Influencing the Development of CPR after Transplantation
Duration of Known Native Kidney Disease
This interval was calculated from the onset of the native disease (first evidence of renal disease occurring at a mean age of 30.7 years; SD=14.4) to the date of initiation of RRT (usually dialysis; mean age =46.0 years; SD=14.6); the mean interval was 15.4 years (SD=11.1). By univariate Cox regression, this covariate had a negative effect, with shorter duration being associated with increased risk of CPR: RR, 0.94 per year; 95% CI, 0.90 to 0.98; β/SEM=−3.28; P=0.001. The significant cutoff value was 16 years. The interval between first RRT and transplantation (mean =2.0 years; SD=2.9) showed no influence on development of CPR.
Induction Treatment
Only 56 patients received induction immunosuppression therapy18: 17 with polyclonal antilymphocyte antibody (ALG) and 39 with an mAb Basiliximab. Recipients not receiving ALG had no significant increased risk of recurrence: RR, 3.22; 95% CI, 0.93 to 11.11; β/SEM=+1.85; P=0.06 (P = NS).
Recipient Age at Transplantation
Increasing age at time of transplantation showed a protective influence on development of CPR: RR, 0.95; 95% CI, 0.93 to 0.98; β/SEM=−3.63; P=0.003. Recipients younger than 49 years old at transplantation (50th percentile) had an increased risk for CPR: RR, 2.85; 95% CI, 1.36 to 5.98; β/SEM=+2.78; P<0.01.
Other Factors
An allograft from a living related donor (only four in our cohort), HLA mismatch, type of calcineurin inhibitor (cyclosporin versus tacrolimus), sex, and cessation of glucocorticoids after transplantation19 had no significant effect on CPR occurrence.
Multivariate Cox Regression Analyses with Significant Clinical and Biologic Covariates
Table 7 shows that the three clinical factors retained no significant influence on CPR occurrence: duration of native kidney disease <16 years, P=0.08; absence of ALG induction therapy, P=0.06; and recipient age at transplantation <49 years, P=0.07. In contrast, normalized serum IgG-autoAb level was an independent risk factor for CPR: RR, 2.96; 95% CI, 1.31 to 6.70; β/SEM=+2.60; P<0.01.
Table 7.
Multivariate Cox regression analysis for prediction of CPR event (n=34) with significant clinical and biologic covariates in the recipients (n=96)
| Covariates | RR (95% CI) | P Value | β/SEM |
|---|---|---|---|
| No ALG induction | 3.16 (0.96 to 10.39) | 0.06 (NS) | +1.89 |
| Native kidney disease duration <16 yr | 2.27 (0.89 to 5.76) | 0.08 (NS) | +1.72 |
| Recipient age <49 yr | 2.17 (0.95 to 4.96) | 0.07 (NS) | +1.84 |
| Norm IgG-autoAb, OD | 2.96 (1.31 to 6.70) | <0.01 | +2.60 |
β/SEM indicates the weight of the covariate with increasing (+) and decreasing (−) the risk; native kidney disease duration indicates the interval from onset of clinical manifestations of native disease to first RRT. Norm, normalized.
Discussion
IgAN frequently recurs after kidney transplantation, and clinically evident disease in the allograft is common.15–17 In this study, we found that the serum level of Gd-IgA1 (the major autoantigen in IgAN) was elevated compared with controls at the time of diagnosis by native kidney biopsy and the time of first renal transplantation. The magnitudes of elevation at native kidney diagnosis and transplantation were similar to those observed at time of native kidney diagnosis in our subgroup of patients with IgAN and absolute renal risk of three (worse prognosis).10 However, this biomarker failed to predict recurrence in the renal allograft. The normalized serum level of IgG-autoAb was significantly elevated at the time of diagnosis and had significantly decreased by the time of transplantation. This biomarker showed significant power of prediction for recurrent disease in the allograft, especially for the earlier recurrences (75% of events occurring before 8.79 years after transplantation). Total IgG anti–Gd-IgA1, calculated by using total serum IgG level, had no predictive value. This finding is possibly related to the variations of total serum IgG levels over time, perhaps due to fluctuations in magnitude of proteinuria in our population (with either native kidney or recurrent IgAN) (Table 2). The serum level of autoantibody of the IgA isotype (both normalized and total) did not change between diagnosis and engraftment and showed no value for prediction of recurrent disease. Nonetheless, the total IgA-autoAb level was associated with allograft loss due to recurrent IgAN.
Several studies16–21 have sought factors to predict recurrence of IgAN after transplantation and its severity. Some investigators20,21 have also assessed the role of autoimmunity biomarkers for recurrence of IgAN after transplantation. In agreement with our findings, Coppo et al.20 found that the serum Gd-IgA1 level did not predict recurrence in patients with manifestations very early after engraftment (mean =2.9 years). In contrast to our results, Berthelot et al.21 found that the serum level of Gd-IgA1 predicted recurrence using a lectin-based assay similar to our assay. There may have been some bias in the selection of patients in their study; patients with recurrent disease had been followed for >12 years after transplantation, whereas those without recurrence had been followed for only 4.7 years. Thus, recurrent IgAN may manifest with longer observation of some of the latter patients. In contrast, we are concordant on the value of serum levels of IgG autoantibody to predict recurrent disease, although the techniques used to measure this biomarker varied. Berthelot et al.21 precipitated circulating immune complexes and then measured IgG-IgA complexes in the precipitate,21 whereas we directly measured the IgG autoantibody in the serum. In addition, they analyzed circulating immune complexes consisting of Gd-IgA1 and CD8922–24 and showed that low serum levels were predictive for recurrence, similar to the reported association between a low level of these complexes and progression of disease in native kidneys.23 They also tested a few patients for these autoimmune biomarkers at 1 year post-transplantation in a longitudinal analysis.
Overall, the factors affecting outcomes for patients with ESRD due to IgAN are similar to those of all recipients receiving a first renal allograft: challenges of surgery, cellular and antibody-mediated rejection, and BK nephropathy that adversely affect renal function and increase the risk of death. In addition, they face the added risk of recurrent disease. The clinical factors associated with recurrence still need further clarification25–27: duration of native kidney disease, age of recipient, induction therapy (with the potential protective role of polyclonal antilymphocyte globulin), maintenance immunosuppressive therapy, living related donor, extent of HLA mismatch, and others.
Our study has several limitations. We were not in a situation to detect subclinical recurrence of IgAN with our clinical practice policy of for-cause allograft biopsies without surveillance biopsies. Also, we did not have longitudinal blood samples at key time points: at clinical onset of the native kidney disease, at initial diagnosis of IgAN (available for only 30 recipients), at initiation of dialysis, and yearly after transplantation.
Although the biomarkers associated with disease-inducing autoimmunity in patients with IgAN may have significant value for prediction of recurrence in renal allografts, the practical effect of our observations remains limited. These results will not modify the current management of patients with IgAN who undergo renal transplantation or the subset who develop recurrent disease. However, with improvement and standardization of the assays and dissemination of these techniques in the future, we hope that a reliable and practical method to identify recipients at increased risk for recurrent disease can be developed. Patients whose recurrent disease can be discovered early will likely benefit from progress in the treatment of native-kidney IgAN.
Concise Methods
Patients
From our original cohort of patients with IgAN and ESRD who had undergone kidney transplantation in Saint Etienne and for whom we had archived frozen serum samples obtained at time of engraftment, we excluded 19 patients who had been previously transplanted and seven others who were transplanted before December 1984 (when cyclosporin became commercially available). The remaining 96 recipients had been engrafted in the interval from January 1985 to December 2007, and maintenance immunosuppression included a calcineurin inhibitor (see supplemental material). Allograft failure was defined as return to dialysis or stage 5 CKD with eGFR<15 ml/min per 1.73 m2. CPR of IgAN was defined as clinically evident disease (proteinuria at least 1 g/d accompanied by microscopic hematuria or, rarely, macroscopic hematuria with eventual hypertension and worsening of renal function with decreasing eGFR) and an allograft biopsy showing mesangial IgA deposits (at least 1+ on a 0–3+ scale) on direct immunofluorescence and characteristic light microscopy features of mesangial hypercellularity and expansion of mesangial matrix. Allograft loss due to recurrent IgAN was defined as predominant advanced glomerular lesions with IgA deposits on initial allograft biopsy or subsequent biopsies to follow progression of allograft damage. At baseline; 6 months; 1, 5, 10, 15, and 20 years after transplantation; and last follow-up/event, we compiled data for weight, systolic and diastolic BP, proteinuria (grams per day or grams per gram urine creatinine), urine RBC (newtons per cubic millimeter), serum creatinine and eGFR by abbreviated Modification of Diet in Renal Disease formula, serum IgA level, current immunosuppressants, and additional medications.
Initial immunosuppression included induction therapy (polyclonal ALG, n=17; basiliximab, n=39; or none, n=40), calcineurin inhibitor (cyclosporin, n=52 or tacrolimus, n=44), prednisone/prednisolone, and an antimetabolite (azathioprine, mycophenolate mofetil, or mycophenolic acid). All recipients received initial triple-drug maintenance immunosuppression therapy. All recipients with CPR had received an ACEI or ARB to control proteinuria (and hypertension, if any). Recipients with recurrent disease and clinical progression received additional treatment with glucocorticoids, similar to our use in native-kidney progressive IgAN. For recipients with recurrent IgAN whose maintenance glucocorticoid agent had been withdrawn, daily prednisolone was restarted.
Controls
We used the same 30 normal healthy controls (20 men and ten women; mean age =45.7 years [SD=12.3]) as in our previous study.10
Laboratory Assays
We had stored serum samples obtained on the day of renal transplantation in all 96 recipients; we retrieved archived serum samples obtained at time of diagnosis of IgAN (first diagnostic native-kidney biopsy) for 30 recipients. All of these samples had been frozen at −70°C until assayed.
Methodology for all assays in this study has been fully described previously.9,10 In brief, serum IgA and IgG were measured by ELISA and expressed in milligrams per milliliter. Serum Gd-IgA1 (autoantigen) was measured by lectin ELISA using Helix aspersa agglutinin, a lectin specific for terminal GalNAc; the binding was expressed in OD units per 1 μg total IgA and then normalized in percentage as the ratio of OD sample to OD 100% (reference was 50 ng Gd-IgA1 [Ale] myeloma protein). Serum total Gd-IgA1 was calculated by multiplying Helix aspersa agglutinin binding in percentage by the IgA concentration and expressed in units per milliliter. Normalized serum IgG autoantibody (IgG-autoAb) was measured by ELISA with Fab fragment of Gd-IgA1 (Ste) myeloma protein (Fab-IgA1)-coated plates; results were expressed in OD per 0.5 μg total IgG. Serum total IgG-autoAb was calculated by multiplying OD per 0.5 μg by the IgG concentration and expressed in units per milliliter. Normalized serum IgA autoantibody (IgA-autoAb) was measured by ELISA with Fab-IgA1–coated plates, followed by detection of captured antibodies, and results were expressed in OD per 1 μg total IgA. Serum total IgA-autoAb was calculated by multiplying OD per 1 μg by the IgA concentration and expressed in units per milliliter.
Statistical Analyses
Descriptive statistics included mean (SD) and median with range values. Comparisons of continuous variables were done by unpaired or paired t test for normally distributed data or the Mann–Whitney U test. P<0.05 was considered statistically significant.
We used receiver operating characteristic curves with C statistics to assess the value of different biomarkers to predict a specific event.
Many events are time dependent, and the influences of different covariates (continuous, nominal, or categorical) were analyzed by Cox regression (first, one by one [univariate analysis] and second, multivariate) to identify significantly independent variables. Kaplan–Meier survival without an event was also used to calculate the cumulative survival at specific intervals after transplantation.
We used two statistical software packages: Statview 5.0 (SAS Institute Inc., Cary, NC) and IBM-SPSS19.1 (IBM-SPSS Inc., Armonk, NY).
Disclosures
J.N. and B.A.J. are cofounders of Reliant Glycosciences, LLC, Birmingham, Alabama.
Supplementary Material
Acknowledgments
This study was supported, in part, by National Institutes of Health grant DK078244 and a gift from the IGA Nephropathy Foundation of America.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016060670/-/DCSupplemental.
References
- 1.Berger J, Hinglais N: [Intercapillary deposits of IgA-IgG]. J Urol Nephrol (Paris) 74: 694–695, 1968 [PubMed] [Google Scholar]
- 2.Wyatt RJ, Julian BA: IgA nephropathy. N Engl J Med 368: 2402–2414, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Berthoux FC, Mohey H, Afiani A: Natural history of primary IgA nephropathy. Semin Nephrol 28: 4–9, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Berger J, Yaneva H, Nabarra B, Barbanel C: Recurrence of mesangial deposition of IgA after renal transplantation. Kidney Int 7: 232–241, 1975 [DOI] [PubMed] [Google Scholar]
- 5.Silva FG, Chander P, Pirani CL, Hardy MA: Disappearance of glomerular mesangial IgA deposits after renal allograft transplantation. Transplantation 33: 241–246, 1982 [PubMed] [Google Scholar]
- 6.Sofue T, Inui M, Hara T, Moritoki M, Nishioka S, Nishijima Y, Moriwaki K, Hayashida Y, Ueda N, Kushida Y, Haba R, Nishiyama A, Kakehi Y, Kohno M: Latent IgA deposition from donor kidneys does not affect transplant prognosis, irrespective of mesangial expansion. Clin Transplant 27[Suppl 26]: 14–21, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthoux F, Mohey H, Maillard N, Mariat C: IgA nephropathy: New aspects in pathophysiology and pathogenesis. EMJ Nephrol 3: 97–103, 2015 [Google Scholar]
- 9.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, Mariat C, Tomino Y, Julian BA, Novak J: Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 23: 1579–1587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K, Gharavi AG, Novak J, Zhang H: The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int 82: 790–796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L: Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 22: 752–761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hariharan S, Peddi VR, Savin VJ, Johnson CP, First MR, Roza AM, Adams MB: Recurrent and de novo renal diseases after renal transplantation: A report from the renal allograft disease registry. Am J Kidney Dis 31: 928–931, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Wang AY, Lai FM, Yu AW, Lam PK, Chow KM, Choi PC, Lui SF, Li PK: Recurrent IgA nephropathy in renal transplant allografts. Am J Kidney Dis 38: 588–596, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ: Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med 347: 103–109, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Ponticelli C, Traversi L, Feliciani A, Cesana BM, Banfi G, Tarantino A: Kidney transplantation in patients with IgA mesangial glomerulonephritis. Kidney Int 60: 1948–1954, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Ponticelli C, Glassock RJ: Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol 5: 2363–2372, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Berthoux F, El Deeb S, Mariat C, Diconne E, Laurent B, Thibaudin L: Antithymocyte globulin (ATG) induction therapy and disease recurrence in renal transplant recipients with primary IgA nephropathy. Transplantation 85: 1505–1507, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Clayton P, McDonald S, Chadban S: Steroids and recurrent IgA nephropathy after kidney transplantation. Am J Transplant 11: 1645–1649, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Coppo R, Amore A, Chiesa M, Lombardo F, Cirina P, Andrulli S, Passerini P, Conti G, Peruzzi L, Giraudi R, Messina M, Segoloni G, Ponticelli C: Serological and genetic factors in early recurrence of IgA nephropathy after renal transplantation. Clin Transplant 21: 728–737, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Berthelot L, Robert T, Vuiblet V, Tabary T, Braconnier A, Dramé M, Toupance O, Rieu P, Monteiro RC, Touré F: Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int 88: 815–822, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Launay P, Grossetête B, Arcos-Fajardo M, Gaudin E, Torres SP, Beaudoin L, Patey-Mariaud de Serre N, Lehuen A, Monteiro RC: Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J Exp Med 191: 1999–2009, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vuong MT, Hahn-Zoric M, Lundberg S, Gunnarsson I, van Kooten C, Wramner L, Seddighzadeh M, Fernström A, Hanson LA, Do LT, Jacobson SH, Padyukov L: Association of soluble CD89 levels with disease progression but not susceptibility in IgA nephropathy. Kidney Int 78: 1281–1287, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Berthelot L, Papista C, Maciel TT, Biarnes-Pelicot M, Tissandie E, Wang PH, Tamouza H, Jamin A, Bex-Coudrat J, Gestin A, Boumediene A, Arcos-Fajardo M, England P, Pillebout E, Walker F, Daugas E, Vrtosvnik F, Flamant M, Benhamou M, Cogné M, Moura IC, Monteiro RC: Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med 209: 793–806, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulay AV, van Walraven C, Knoll GA: Impact of immunosuppressive medication on the risk of renal allograft failure due to recurrent glomerulonephritis. Am J Transplant 9: 804–811, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Moroni G, Longhi S, Quaglini S, Gallelli B, Banfi G, Montagnino G, Messa P: The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol Dial Transplant 28: 1305–1314, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Wyld ML, Chadban SJ: Recurrent IgA nephropathy after kidney transplantation. Transplantation 100: 1827–1832, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.